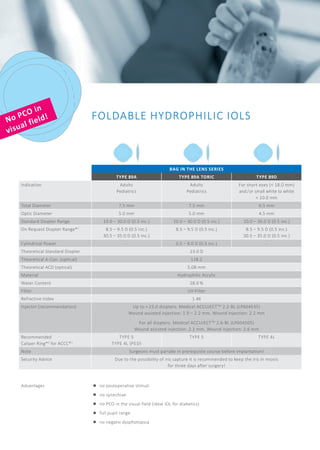
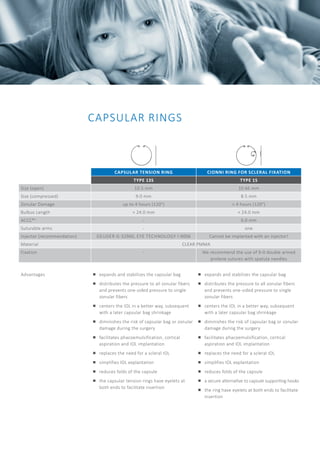
The document discusses the bag-in-the-lens (BIL), an intraocular lens designed to prevent secondary opacification, with clinical studies beginning in 2000. It outlines the training required for surgeons to become certified BIL users and lists various types of ophthalmic implants suitable for short eyes, detailing their specifications and advantages. Additionally, it includes recommendations for surgical techniques and post-operative care to optimize outcomes for patients with specific eye conditions.