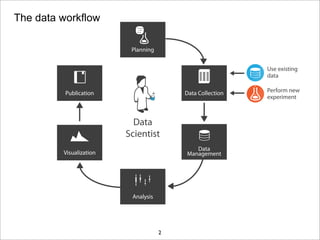
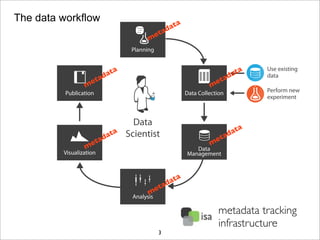
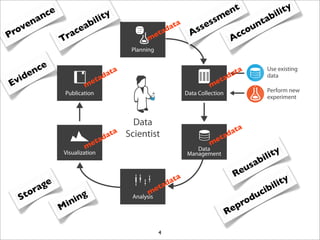
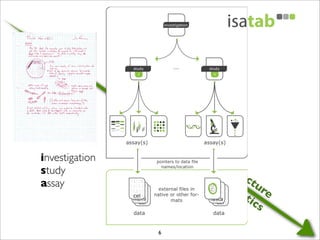
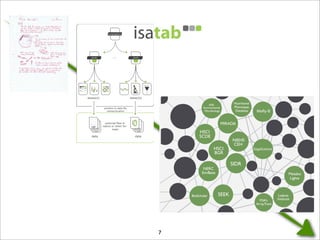
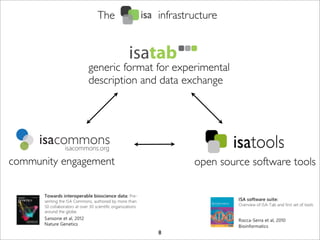
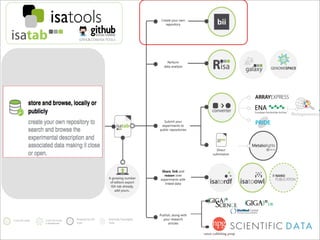
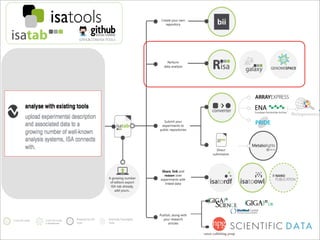
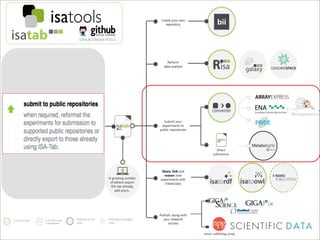
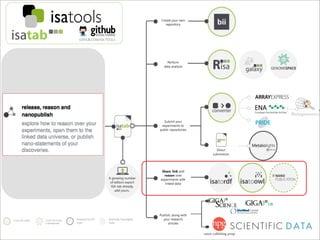
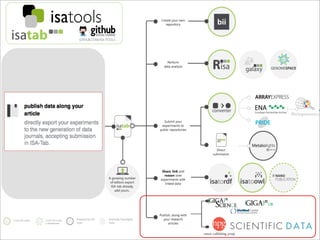
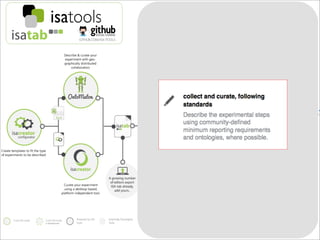
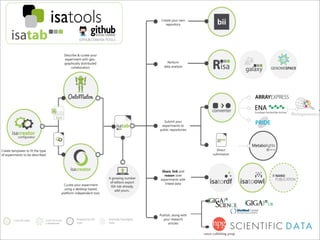
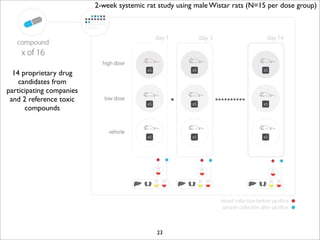
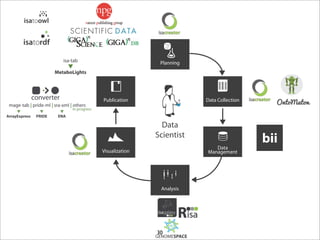
The document discusses the ISA infrastructure, which provides a standardized format (ISA-TAB) for experimental metadata and data exchange. It can be used across various domains like toxicology, systems biology, and nanotechnology. The Risa R package integrates experimental metadata with analysis and allows updating metadata. Nature Scientific Data is a new publication for describing valuable datasets. The ISA framework has been adopted by over 30 public and private resources and is growing in use for facilitating reuse of investigations in various life science domains. Toxicity examples include EU projects on predictive toxicology and a rat study of drug candidates. Questions can be directed to the ISA tools group.