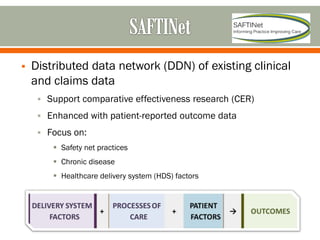
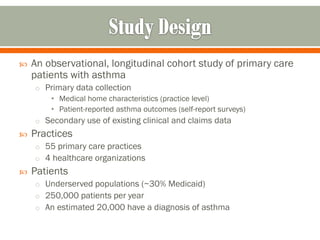
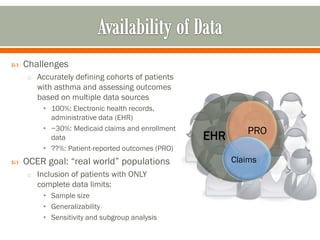
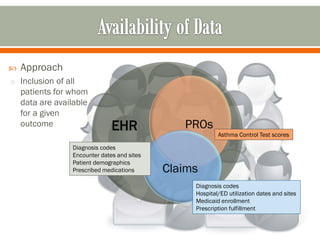
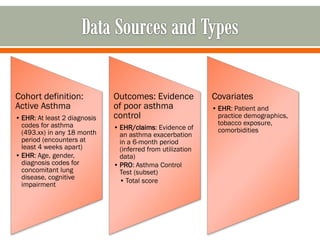
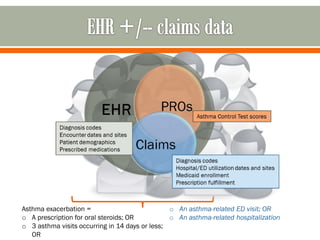
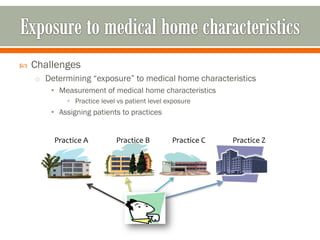
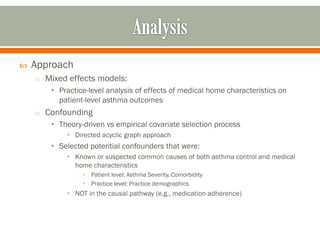
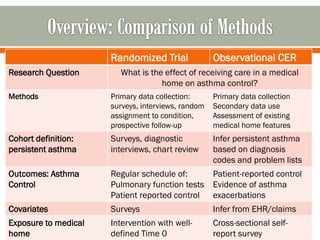
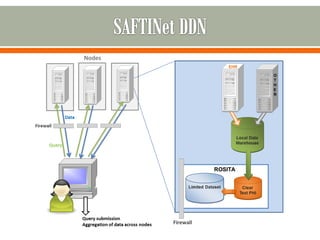
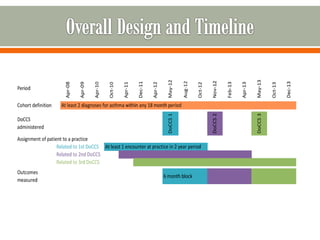
The document outlines a study by the SAFTINET team focusing on data networks for comparative effectiveness research related to asthma control among underserved populations. It emphasizes the relationship between medical home characteristics and asthma management, detailing methodologies including observational cohort studies, data collection methods, and analytical approaches to address challenges in defining patient cohorts and outcomes. Additionally, it highlights the key partnerships involved in the research and addresses the goal of improving healthcare delivery through enhanced data utilization and patient-centered practices.