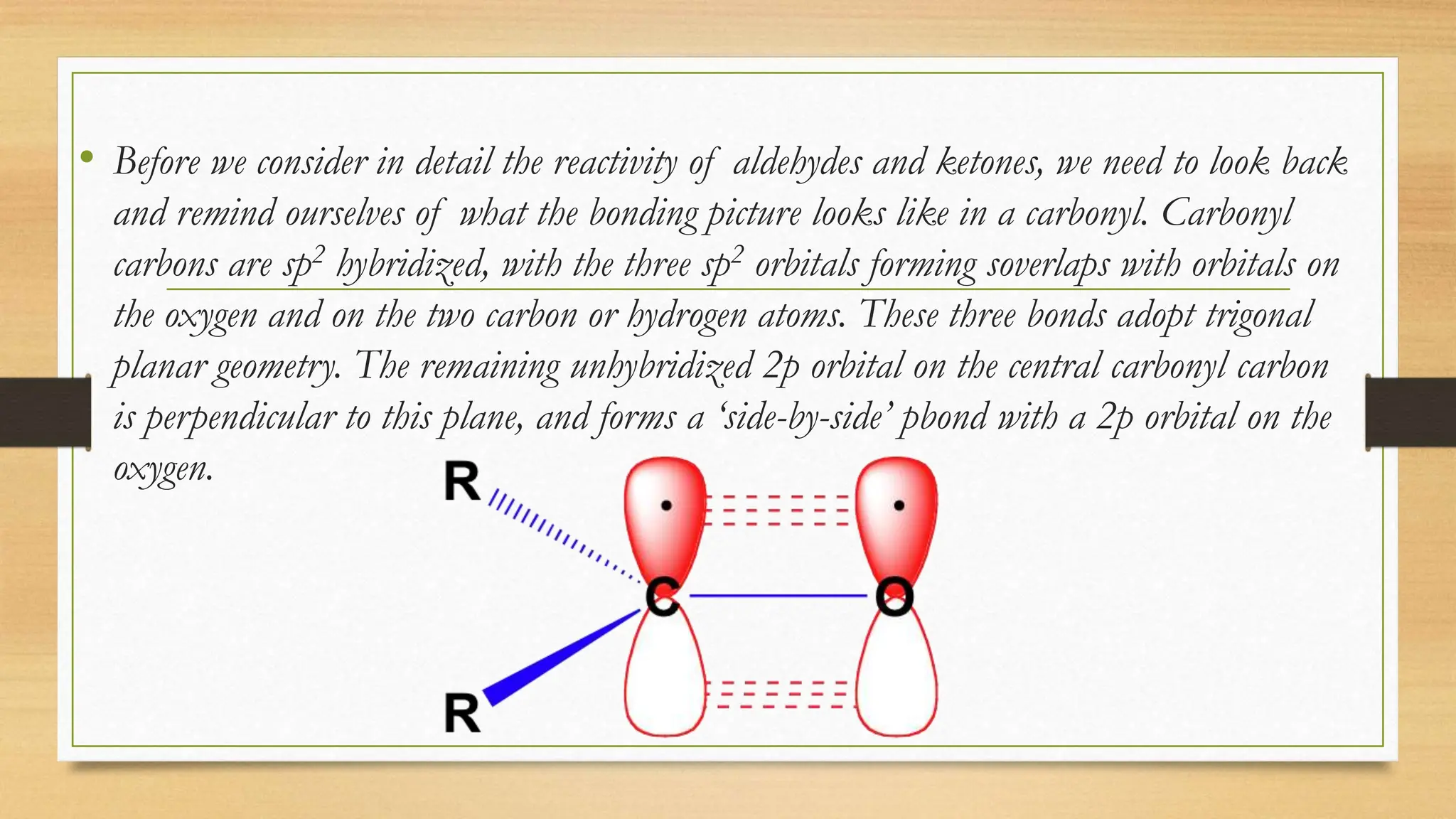
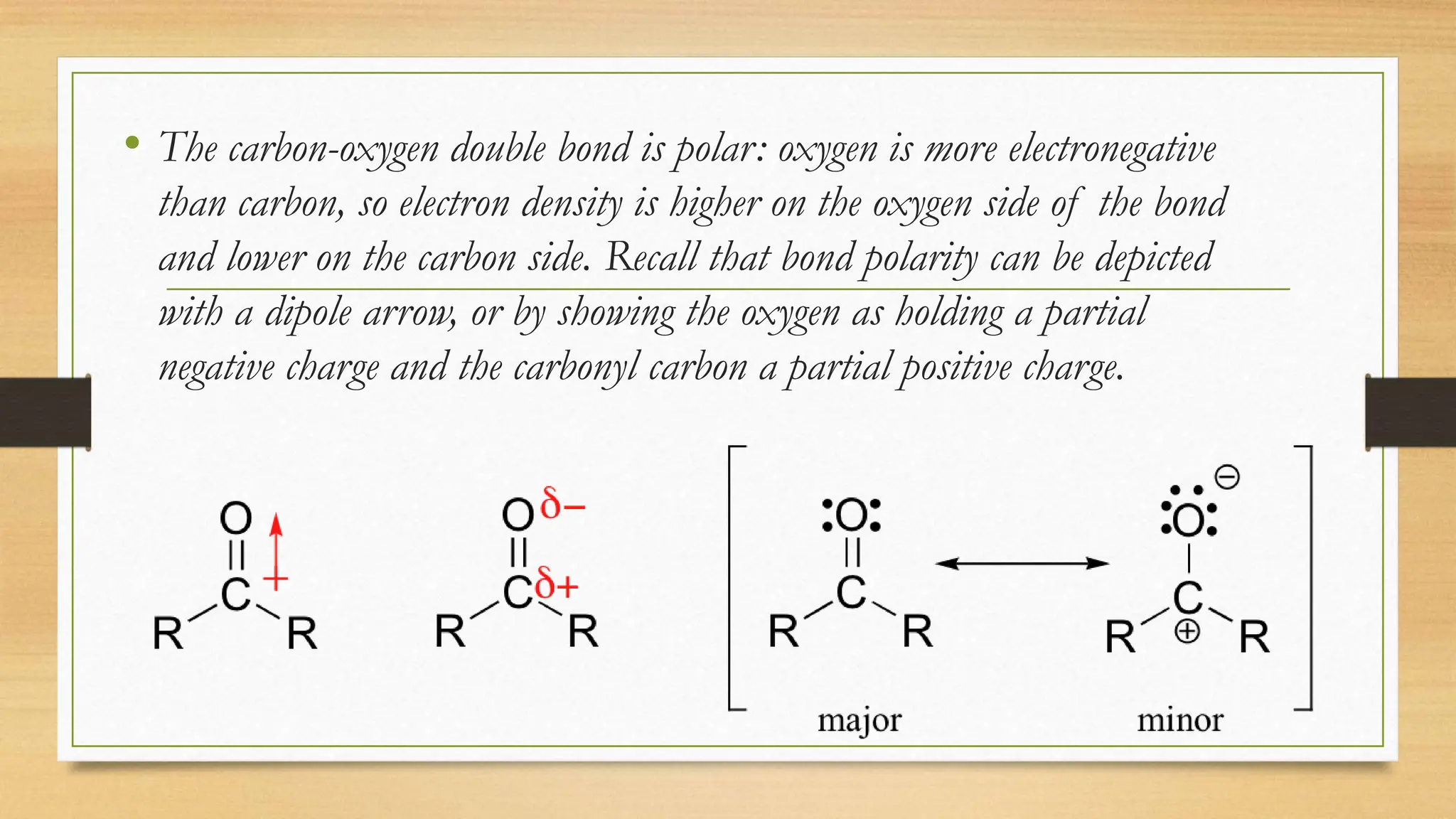
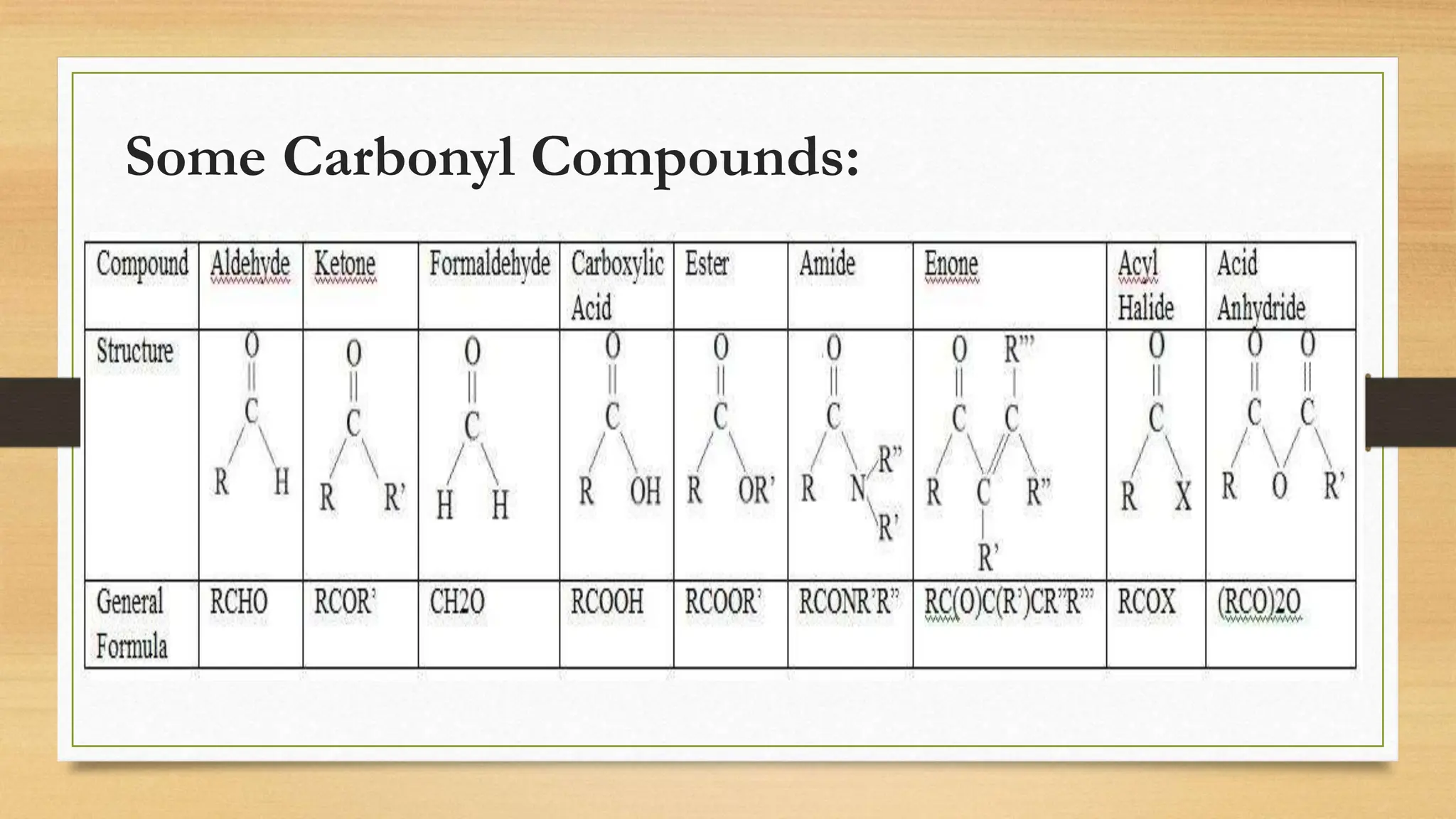
The document discusses the chemistry of carbonyl groups, which consist of a carbon atom double-bonded to an oxygen atom, leading to functional groups like aldehydes and ketones. It explains the reactivity of carbonyls due to their polar nature and resonance forms, facilitating nucleophilic attacks. The document also highlights the bonding characteristics of carbonyl carbons and their geometric structure.
![Introduction:
•A carbonyl group is a chemically organic functional group
composed of a carbon atom double-bonded to an oxygen
atom --> [C=O] The simplest carbonyl groups
are aldehydes and ketones usually attached to another carbon
compound. These structures can be found in many aromatic
compounds contributing to smell and taste.](https://image.slidesharecdn.com/newmicrosoftpowerpointpresentation-240603143120-1f81116b/75/New-Microsoft-PowerPoint-Presentation-pptx-2-2048.jpg)