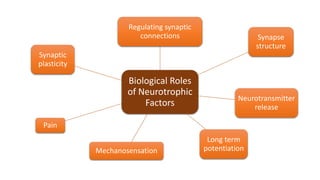
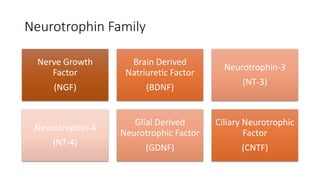
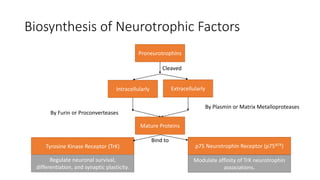
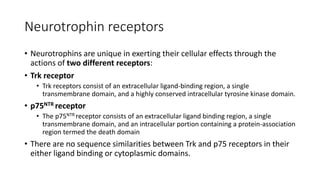
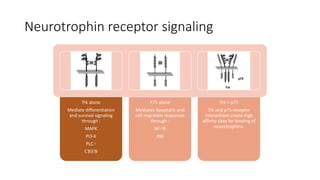
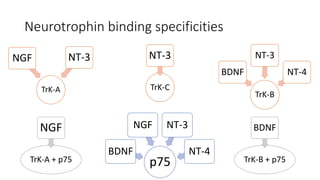
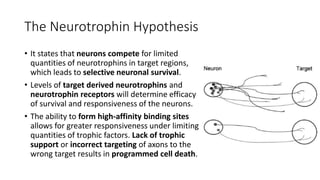
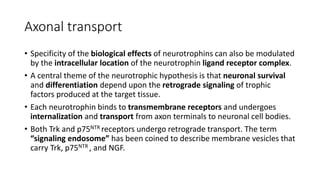
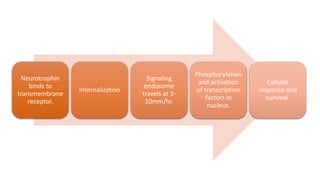
Neurotrophic factors such as NGF, BDNF, and NT-3 regulate neuronal proliferation, differentiation, survival and death. They play roles in synaptic plasticity and neuronal circuit formation. Alterations in neurotrophic factor signaling have been implicated in neurodegenerative and psychiatric disorders. While direct administration of neurotrophic factors has faced challenges, future therapies may aim to enhance endogenous neurotrophic factor levels or activity through other receptor systems to treat these conditions.