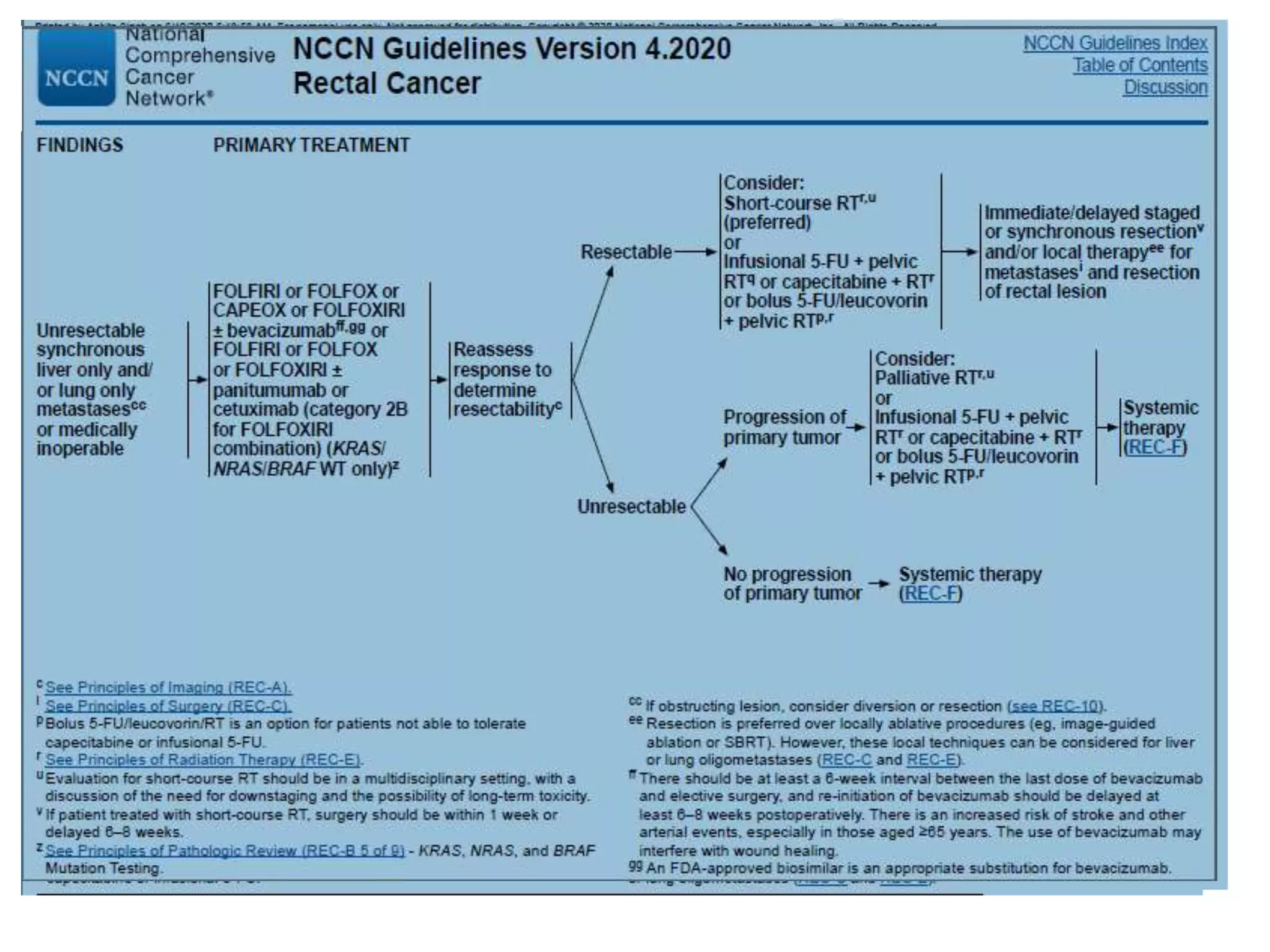
- Several studies have shown that neoadjuvant therapy decreases local recurrence rates in colorectal cancer when compared to surgery alone. One study showed a statistically significant decrease in local recurrence with the addition of chemotherapy to preoperative radiotherapy.
- Evidence indicates that long-course chemoradiotherapy, induction chemotherapy followed by long-course chemoradiotherapy, and short-course radiotherapy are the three accepted neoadjuvant approaches, with long-course chemoradiotherapy being the most commonly used currently. Short-course radiotherapy has also shown non-inferior oncologic outcomes compared to long-course chemoradiotherapy in some studies.