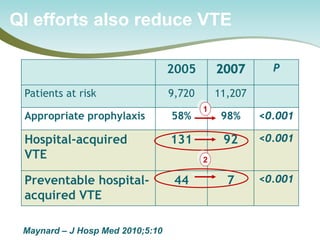
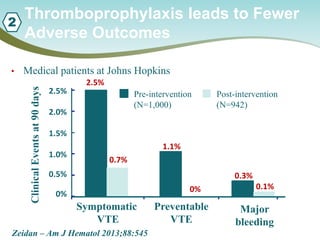
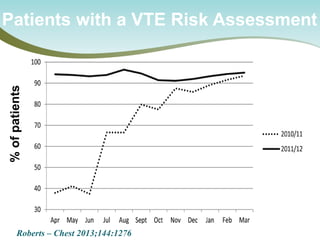
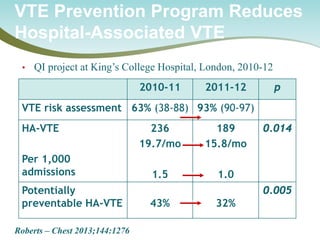
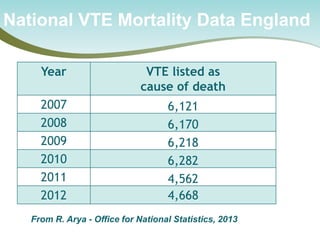
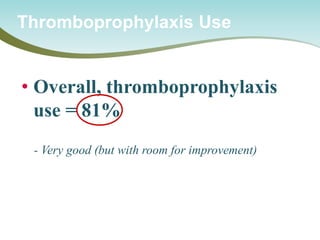
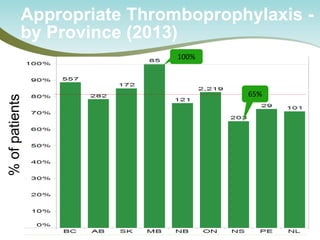
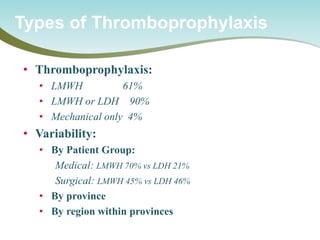
The document outlines a national call to action for a venous thromboembolism (VTE) audit aimed at improving patient safety by focusing on thromboprophylaxis implementation, given that 60% of VTE cases are hospital-acquired. It includes details about the audit's purpose, procedures for participation, and the importance of measuring compliance with safety standards. The document also provides data from previous audits, highlighting areas for improvement and the positive effects of quality improvement efforts on VTE prevention.












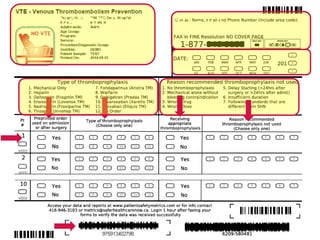
![•
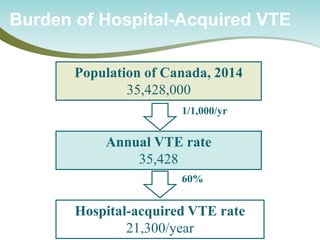
All patients admitted to all 163 NHS trusts, 2010-12
•
Mandatory reporting of use of the VTE risk tool
Use of the UK National VTE Risk Assessment Tool
Lester – Heart – 2013;99:1734
Rate of VTE risk assessments performed [IQR]
100%
0%
50%
July 2010
March 2012
51% [27,71]
93% [91,96]
4](https://image.slidesharecdn.com/nationalcall-vte-2014-09-10-140910103653-phpapp01/85/Canadian-VTE-Audit-Information-Call-22-320.jpg)
![•
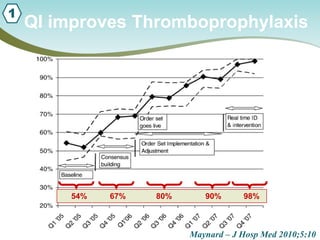
All 4 million patients admitted to all 163 NHS hospital trusts >3 days, 2010-12
Hospital-Acquired Fatal VTE is Reduced in Adherent Hospitals
Lester – Heart – 2013;99:1734
Fatal VTE <90 days after hospital discharge
Rel Risk for hospitals with VTE risk assessment >90% vs <90%
All
0.85 [0.75-0.96; p=0.01]
Post-discharge
0.81 [0.67-0.79; p=0.03]
Achieving >90% VTE risk assessment is associated with significant lower VTE mortality](https://image.slidesharecdn.com/nationalcall-vte-2014-09-10-140910103653-phpapp01/85/Canadian-VTE-Audit-Information-Call-23-320.jpg)

















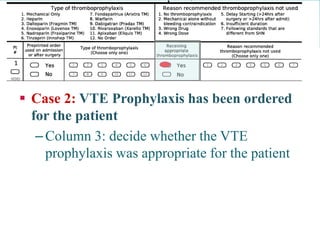









![PS Metrics can be used to support:
•
Small and Large Scale Improvement Initiatives
•
Roll Up or Drill Down Reports [e.g. Unit Site Program Corporation Region Province Node National]
•
Produce automated run charts
•
Reporting for accountability
•
Possible to customize indicators to meet provincial, regional and local reporting needs
Potential applications of the system](https://image.slidesharecdn.com/nationalcall-vte-2014-09-10-140910103653-phpapp01/85/Canadian-VTE-Audit-Information-Call-57-320.jpg)