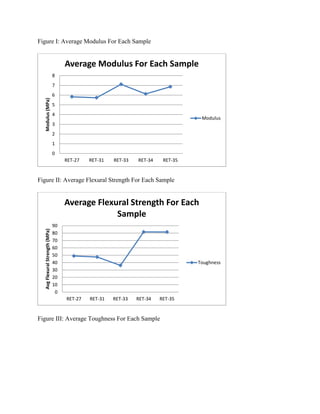
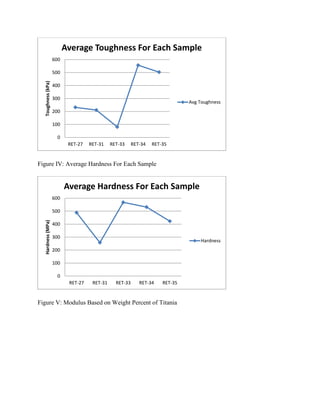
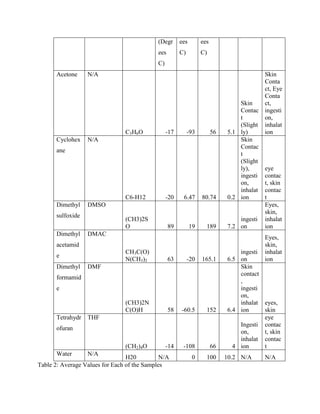
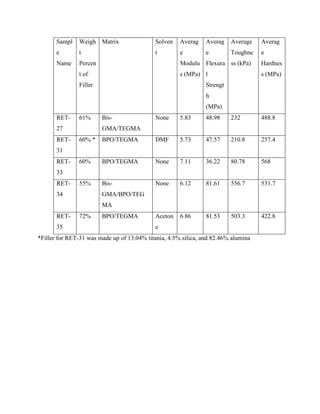
The document discusses research into developing dental composite fillings with improved mechanical properties and aesthetics. Five composite samples were tested with variations in polymer matrix composition, filler material and weight percentage, and use of solvents. Sample RET-27 served as the control with 61% titania filler and a BisGMA/TEGDMA matrix. RET-31, using multiple fillers and a solvent, performed poorly. RET-33 had the highest modulus but lowest strength, using a TEGMA/BPO matrix and 60% titania. RET-34 and RET-35 tested variations in matrix and filler percentage, showing the effects of these parameters on mechanical properties. The goal is to optimize a composite with performance comparable to natural