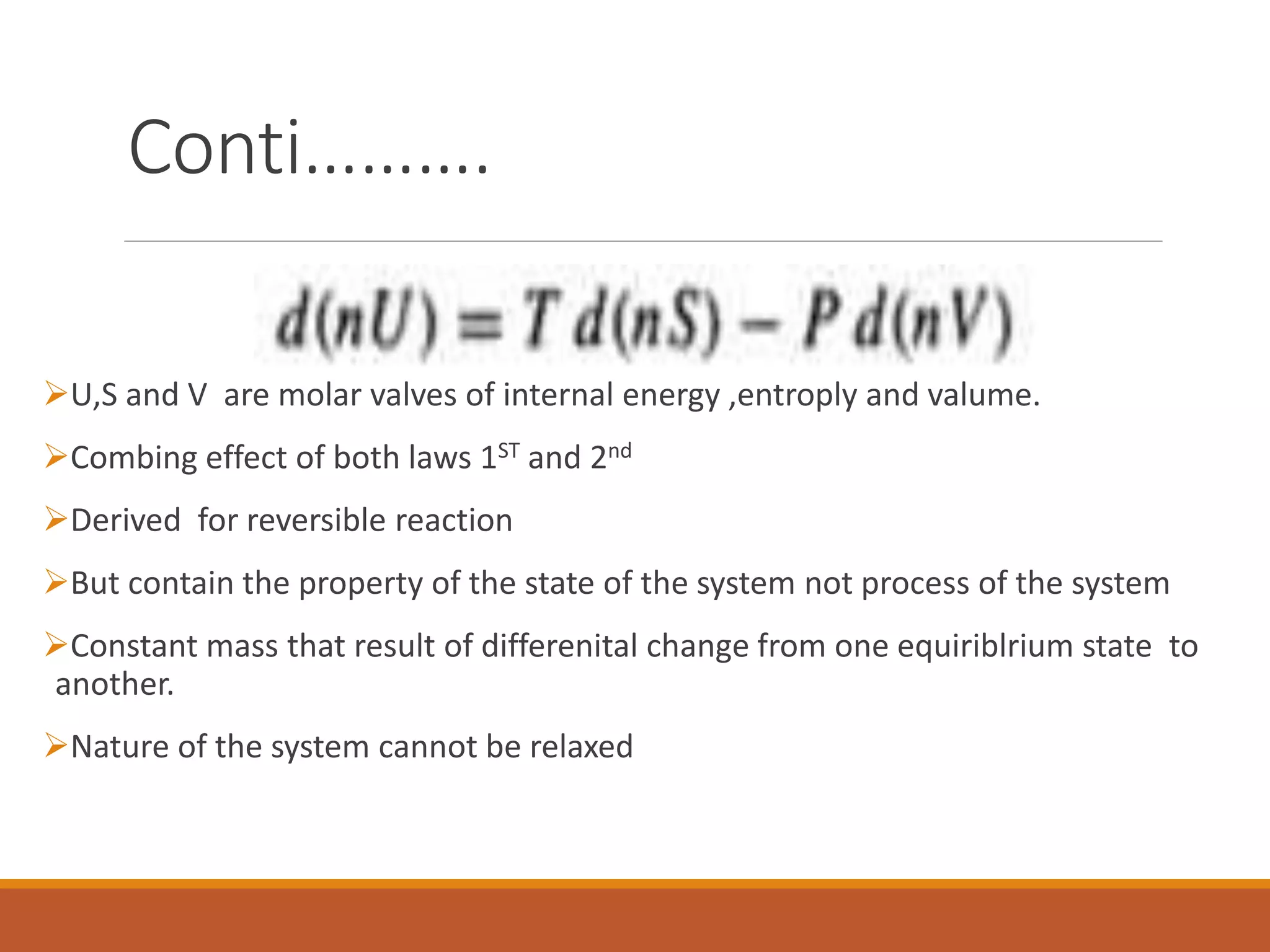
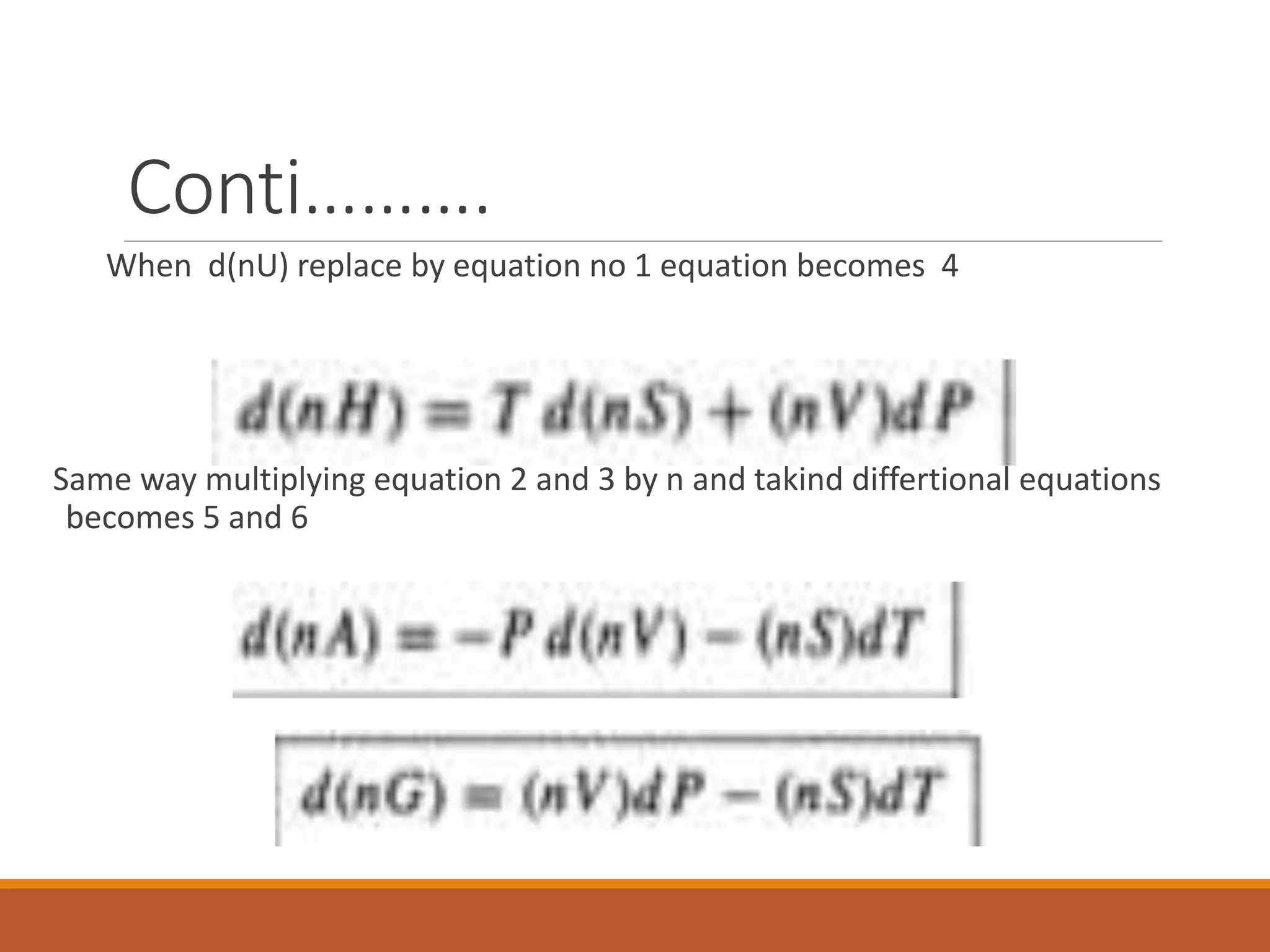
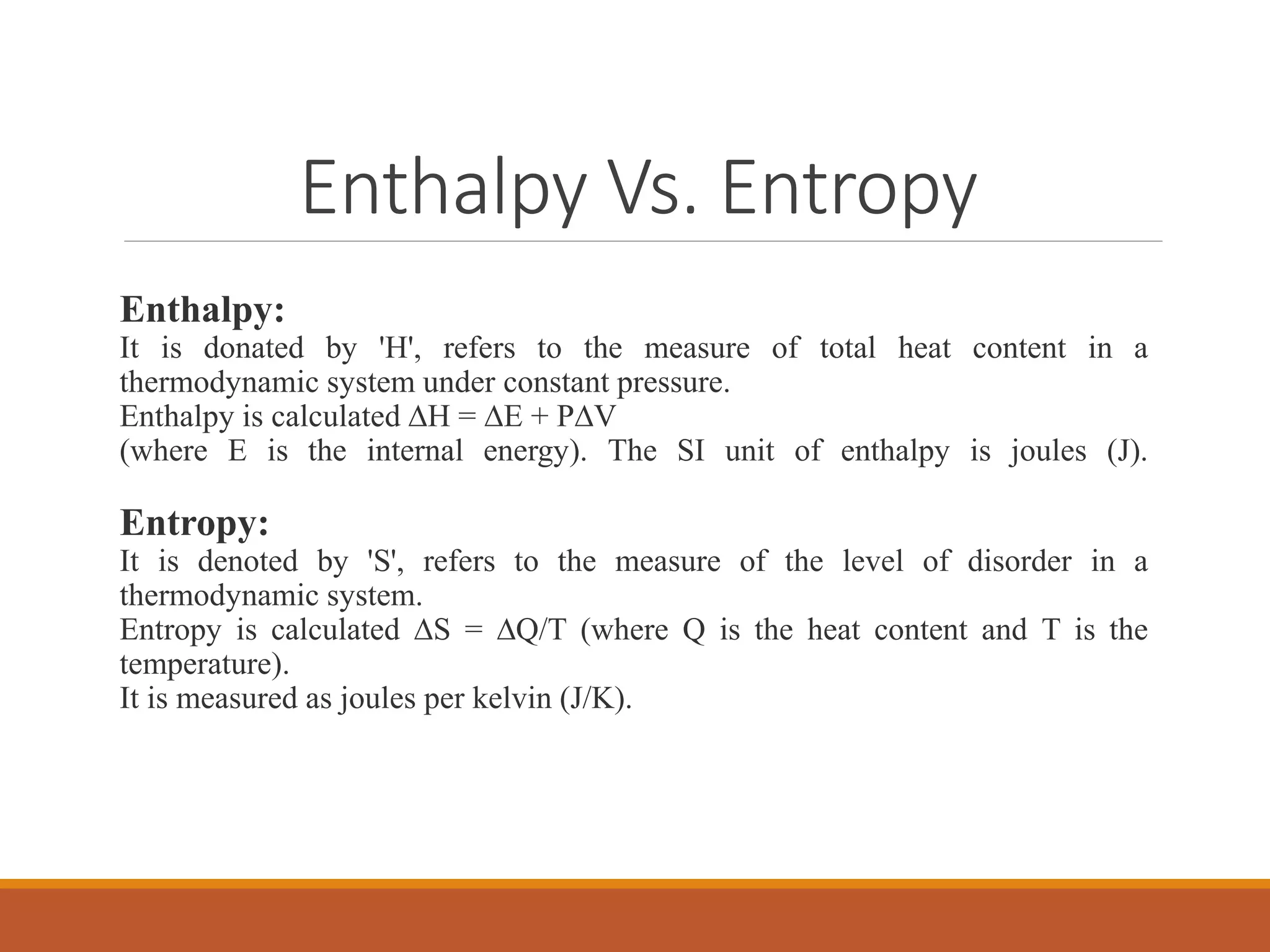
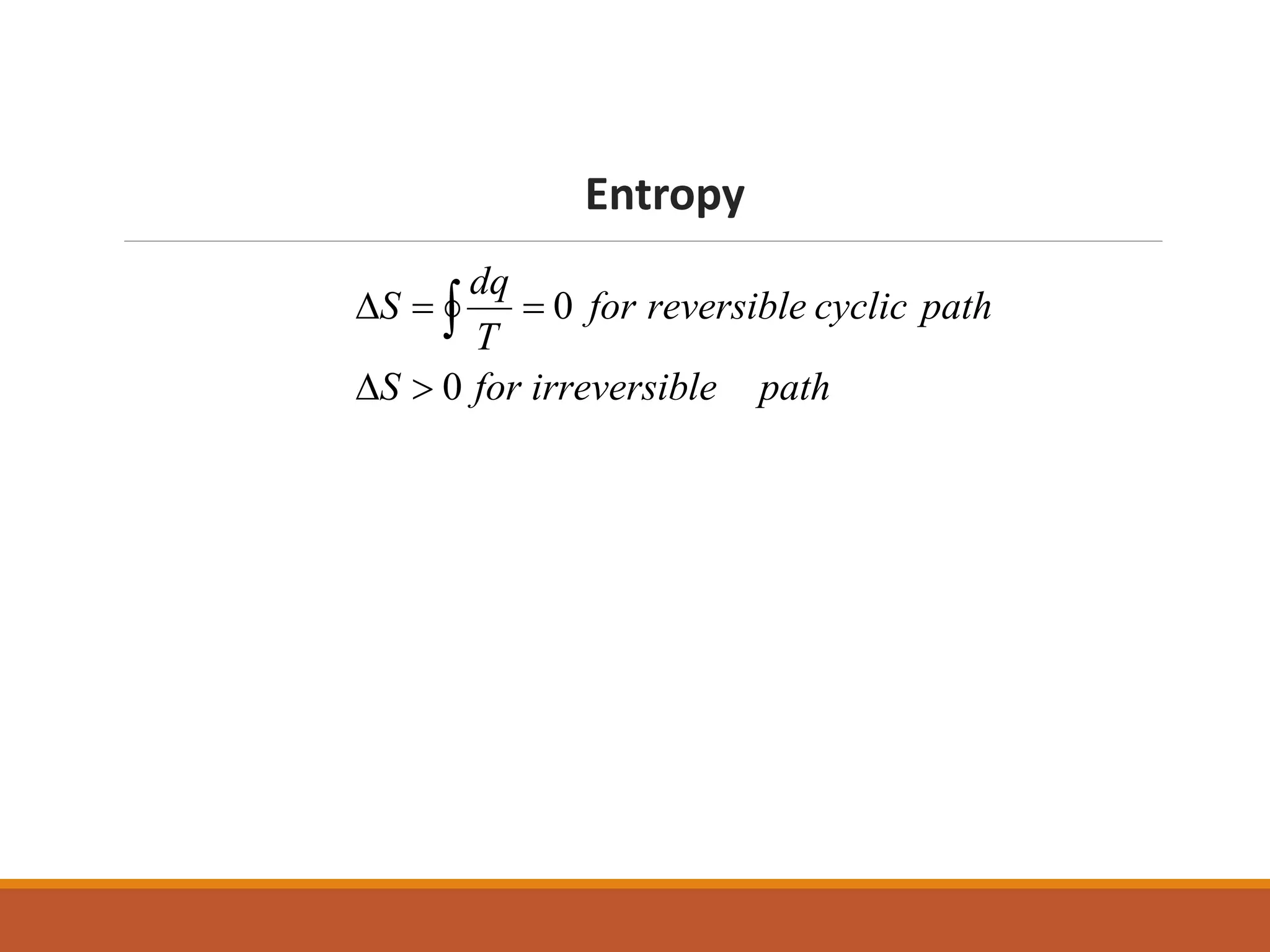
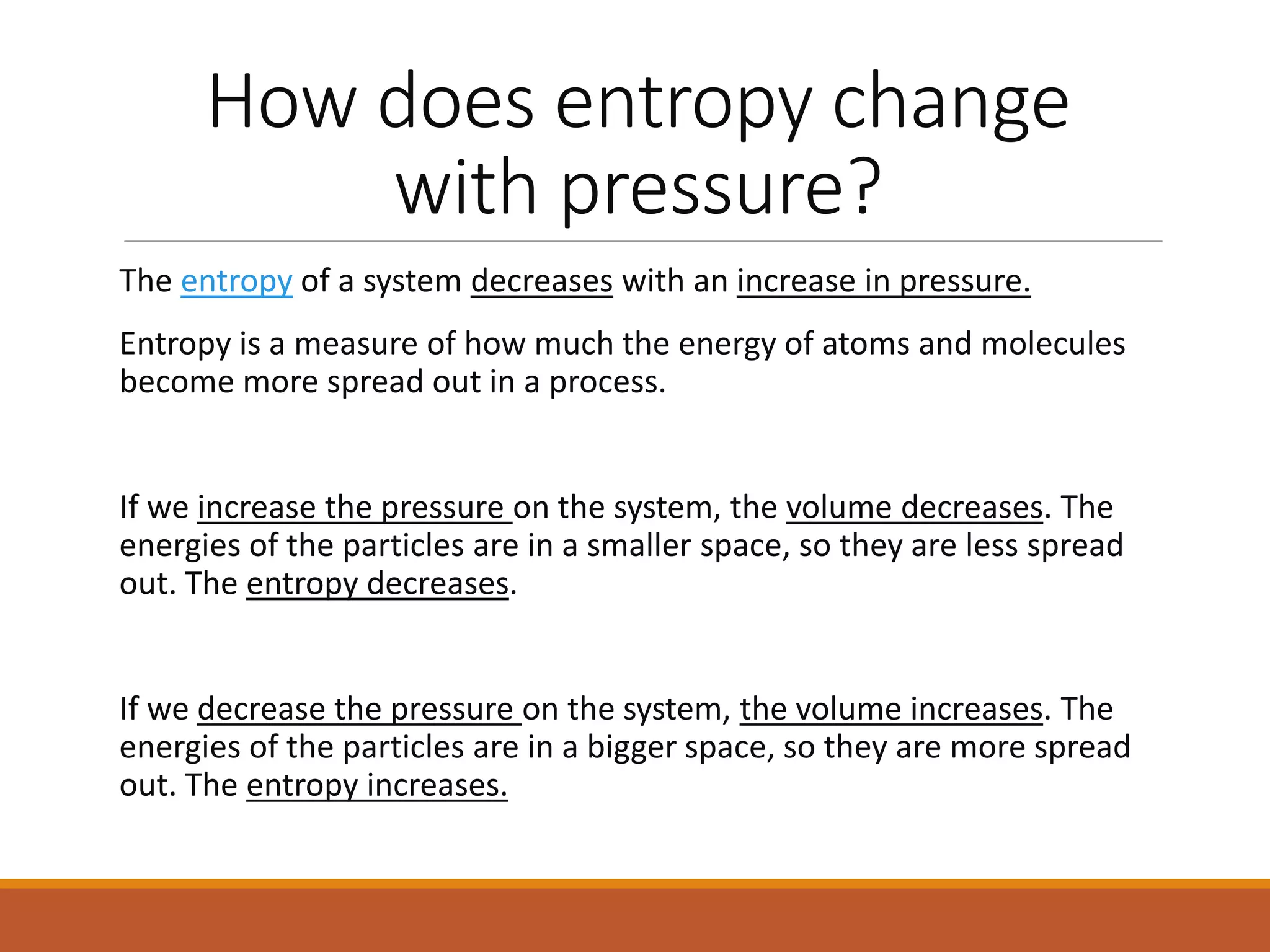
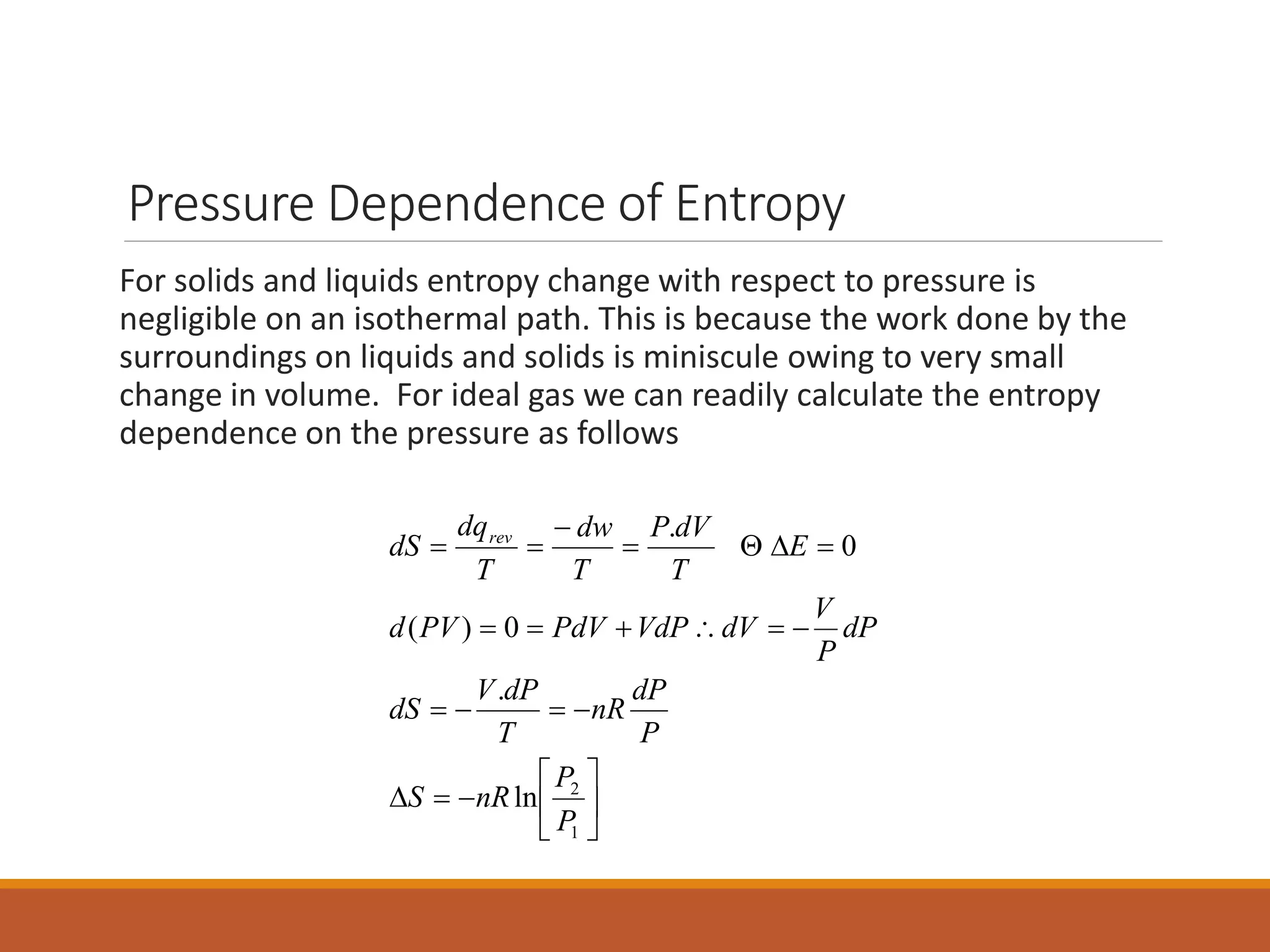
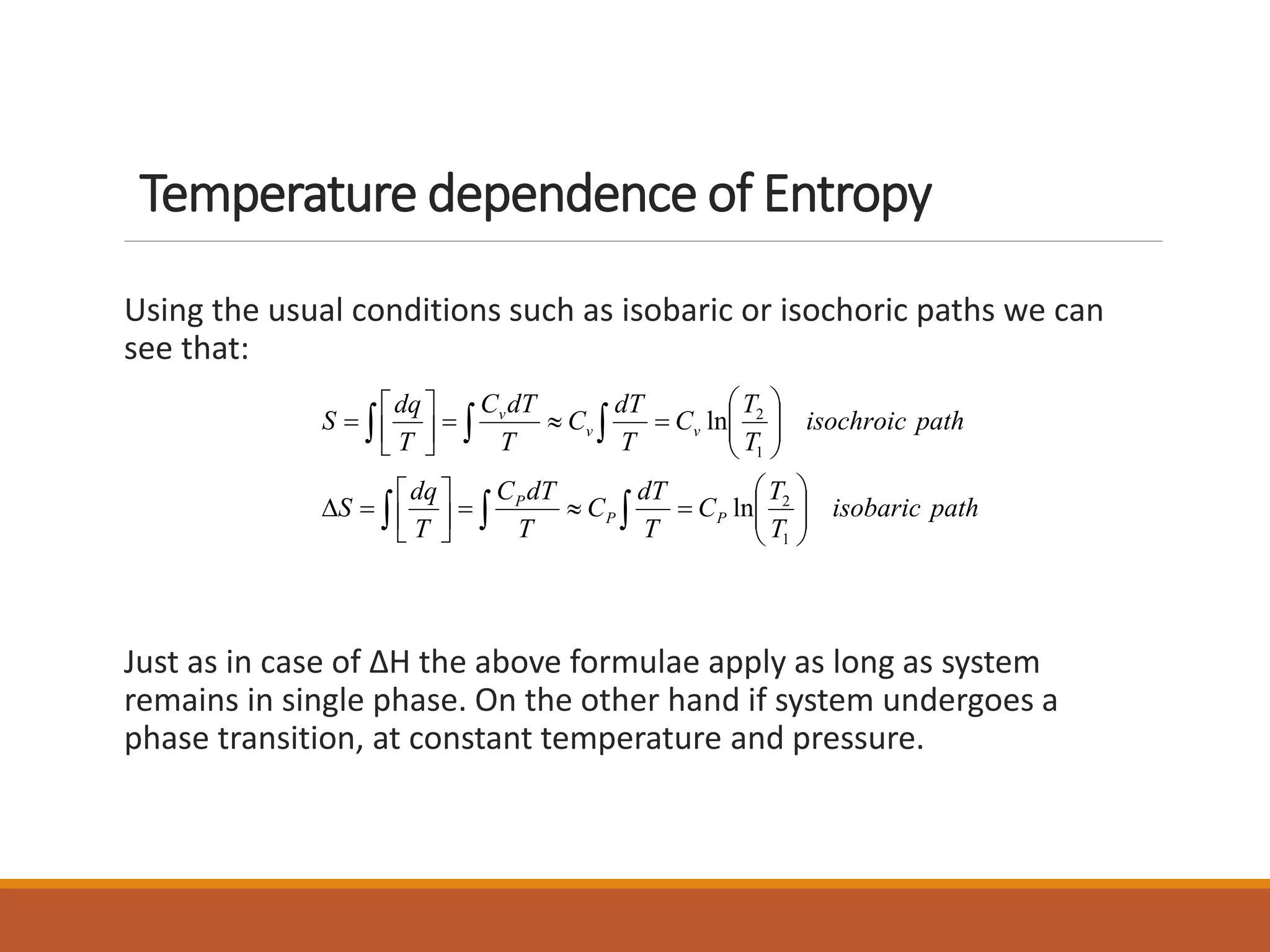
This document discusses thermodynamic properties and relationships for homogeneous phases. It defines key concepts like internal energy, enthalpy, entropy, and Gibbs free energy. Equations are derived relating these properties to temperature and pressure. The relationships show that entropy decreases with increasing pressure as particles are confined to a smaller space, reducing disorder. Gibbs free energy can be used to predict spontaneity of reactions according to the second law of thermodynamics.























![dU= (
𝜕𝑈
𝜕𝑇
)v dT+ (
𝜕𝑈
𝜕𝑉
)t dV
dU= Cv dT+[T (
𝜕𝑆
𝜕𝑉
)-P]dV
dS=(
𝜕𝑆
𝜕𝑇
)v dT+ (
𝜕𝑆
𝜕𝑉
)t dV
dS= CV/TdT+ (
𝜕𝑃
𝜕𝑇
)vdV
As we know
(
𝜕𝑃
𝜕𝑇
)v=
𝛃
𝚔](https://image.slidesharecdn.com/ibad-170205160801/75/nasir-31-2048.jpg)













