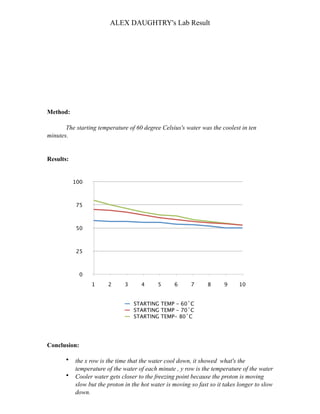
The student, Alex Daughtry, conducted an experiment to determine which starting water temperature (60, 70, or 80 degrees Celsius) would cool down the most in 10 minutes. They found that water starting at 60 degrees Celsius cooled down the most. Their method controlled variables like container size and room temperature. They concluded that cooler water gets closer to the freezing point faster since protons move more slowly, causing hotter water to take longer to cool down. Impurities in the water could affect results due to the unsolved Mpemba effect.