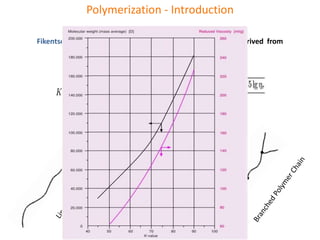
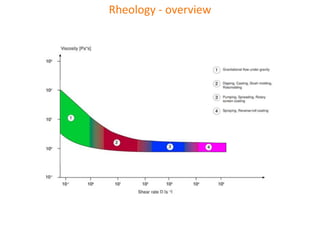
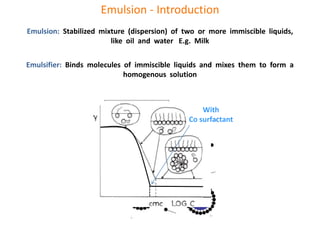
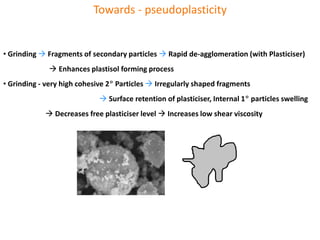
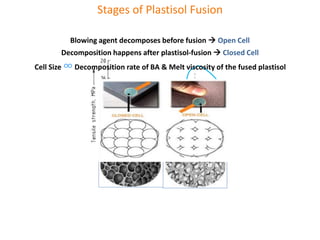
The document discusses polyvinyl chloride (PVC) paste polymerization and plastisols. It describes suspension, emulsion, and microsuspension polymerization methods. Plastisol applications include leather cloth, textiles, coatings, and more. India's PVC paste resin demand and imports are growing about 7% annually. Primary PVC particles from emulsion polymerization are 0.1-2 micrometers and form porous secondary particles after spray drying. Plastisol rheology depends on particle size distribution and residual emulsifiers. Upon heating, plasticizers diffuse into PVC particles to form a homogeneous material, with complete fusion providing maximum properties.