This document provides guidance on specimen management for nursing students. It discusses the importance of proper specimen collection, handling, transportation and storage to ensure quality laboratory test results. Key points include:
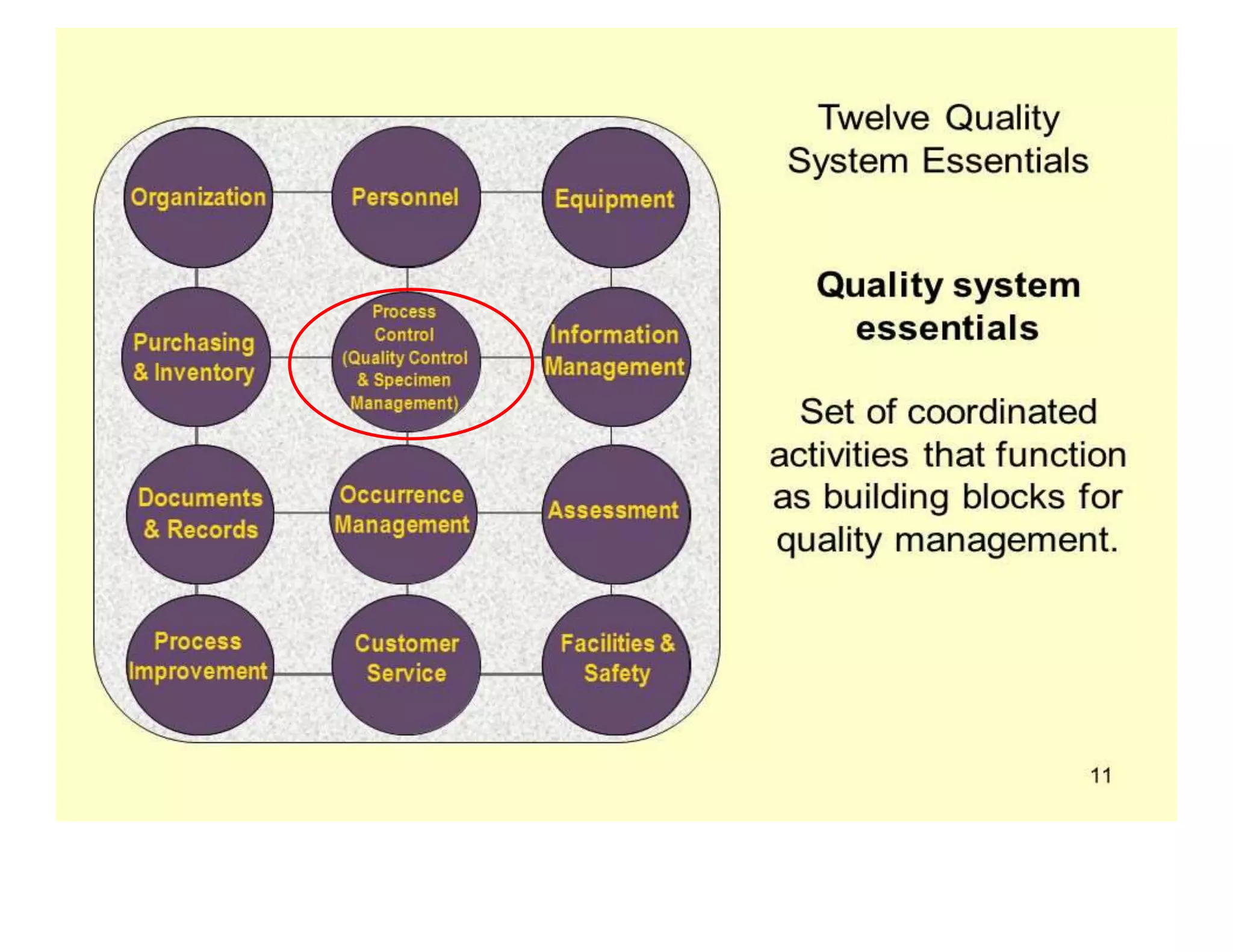
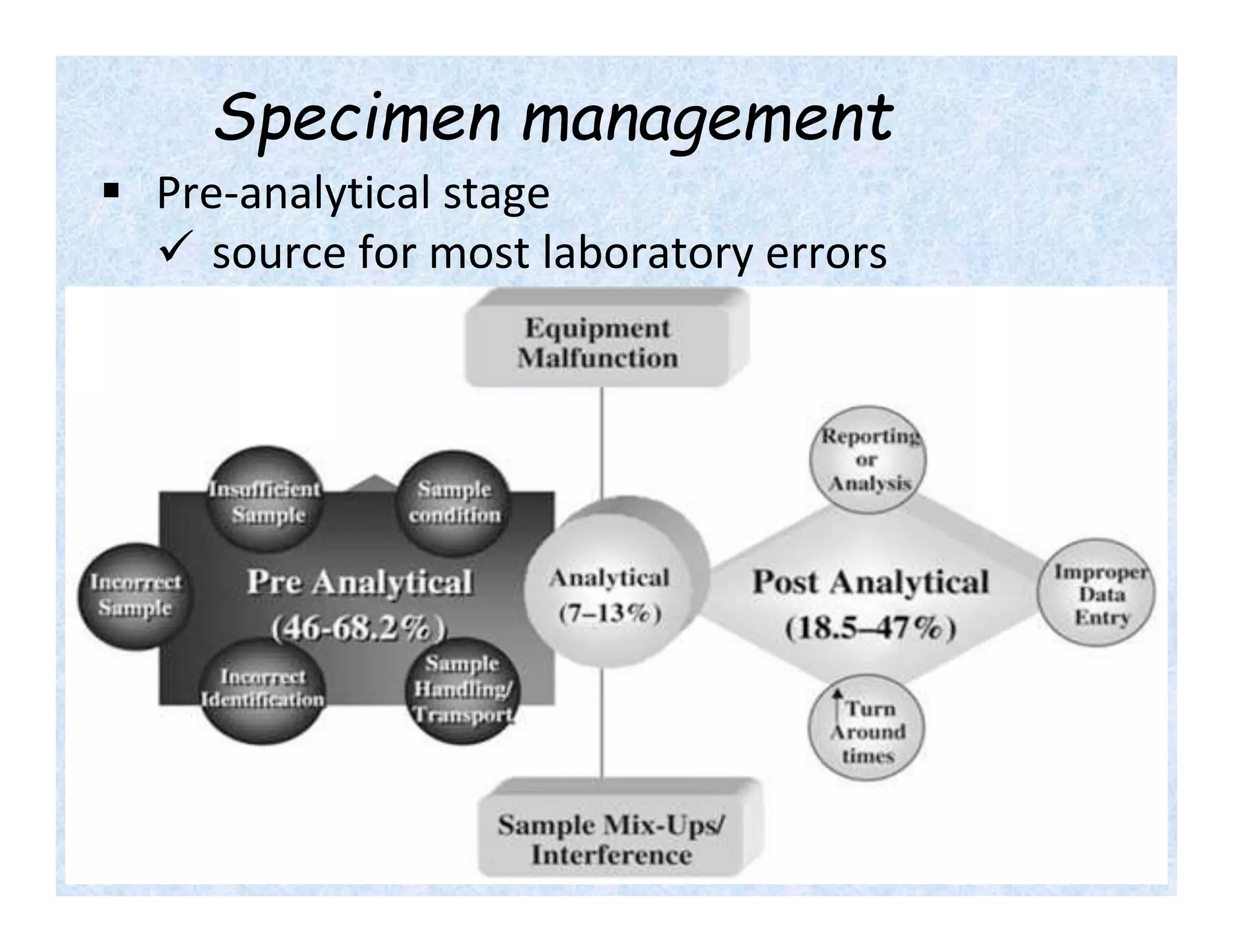
- Specimen management is important to reduce laboratory errors and involves proper instructions, collection, handling, transportation and storage.
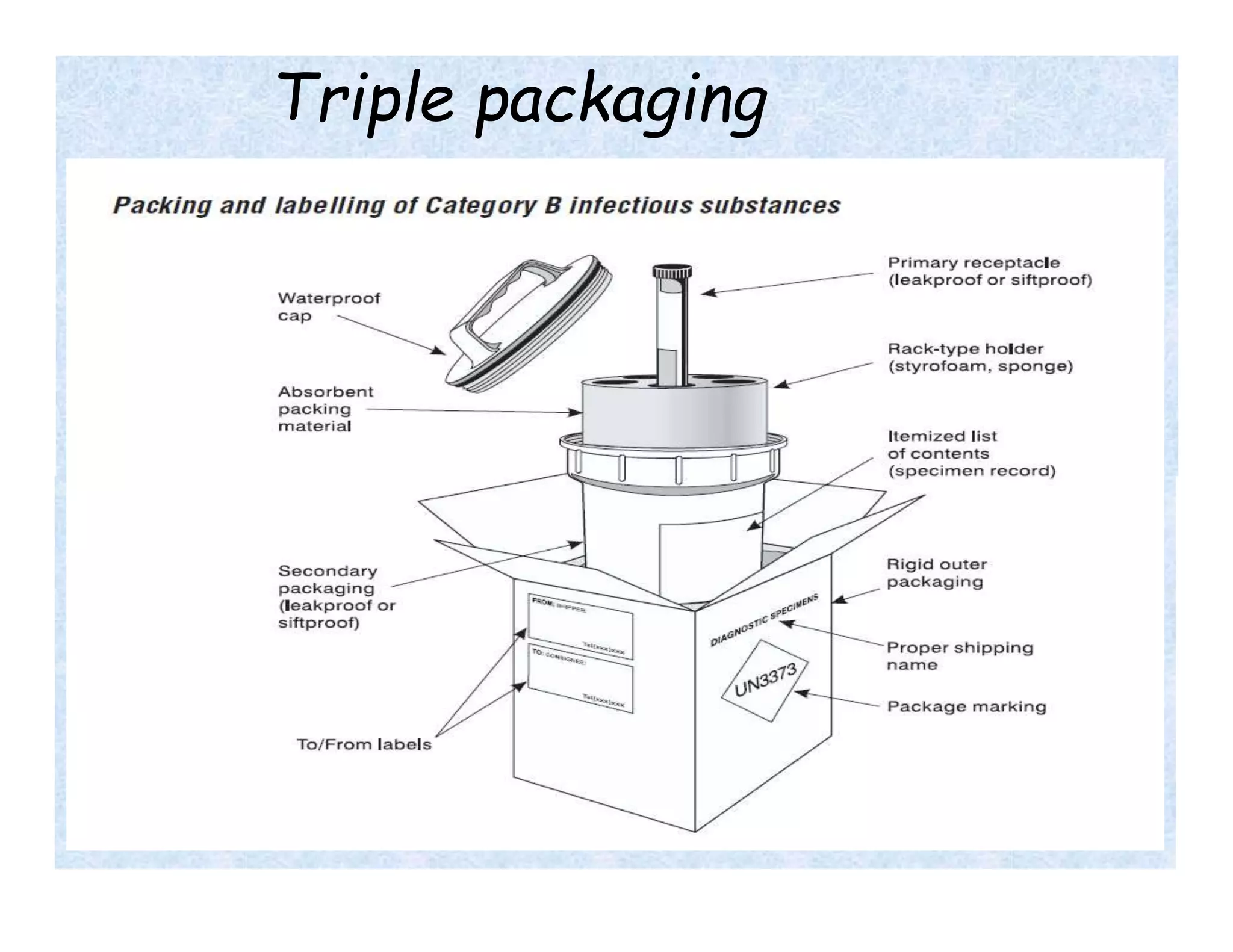
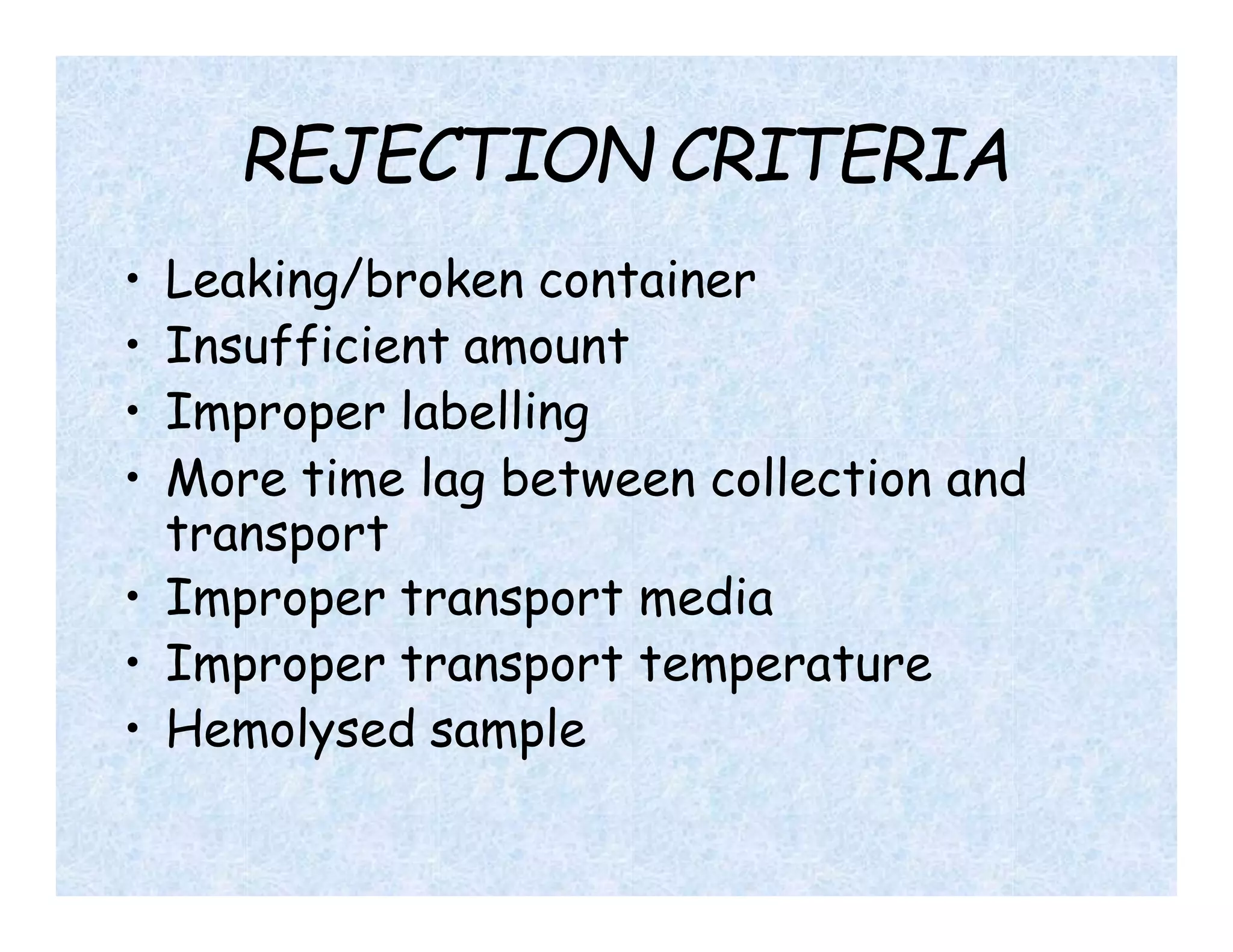
- Universal precautions should be followed when collecting all specimens to prevent exposure to biohazards. Samples must be properly labeled and packaged for transport.
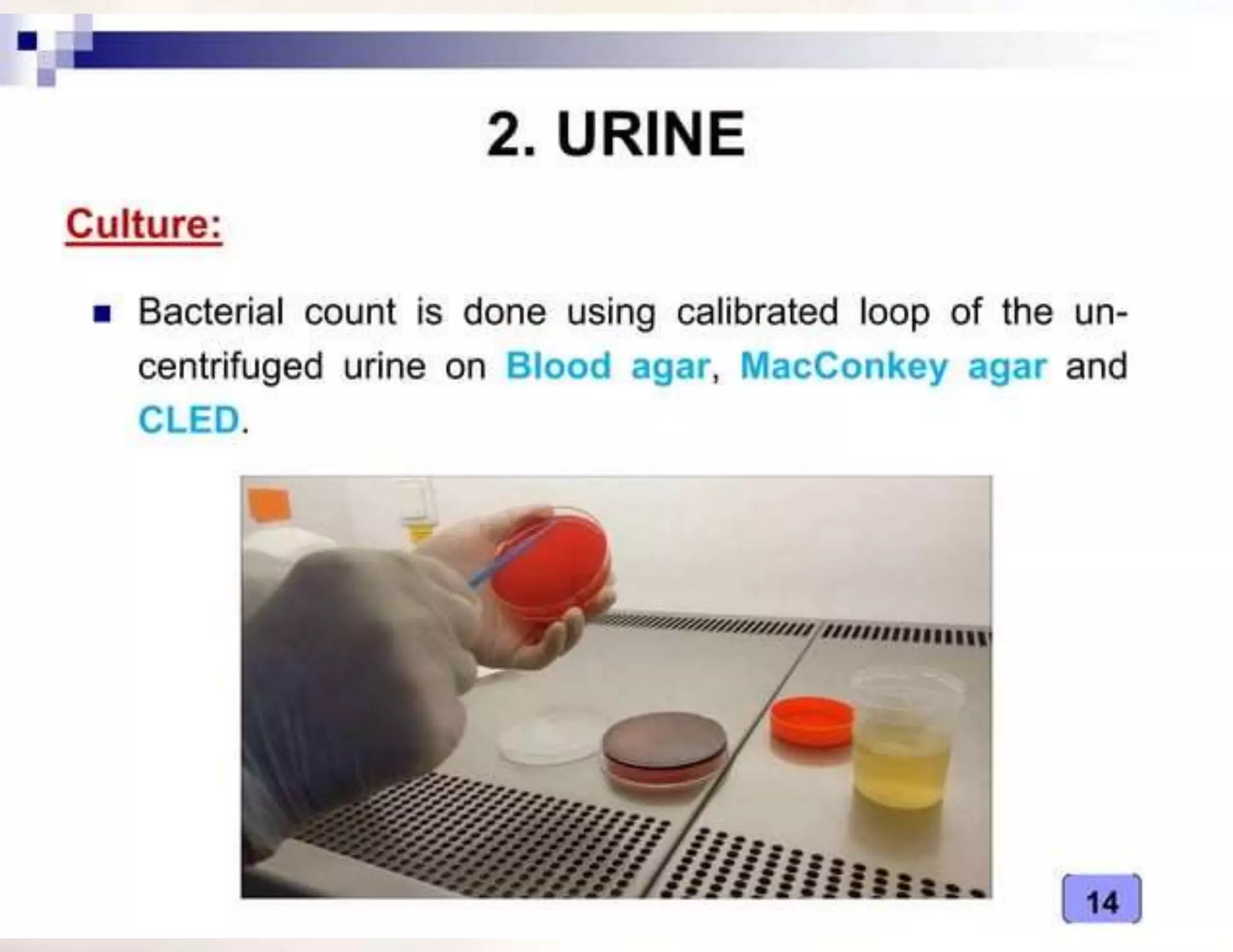
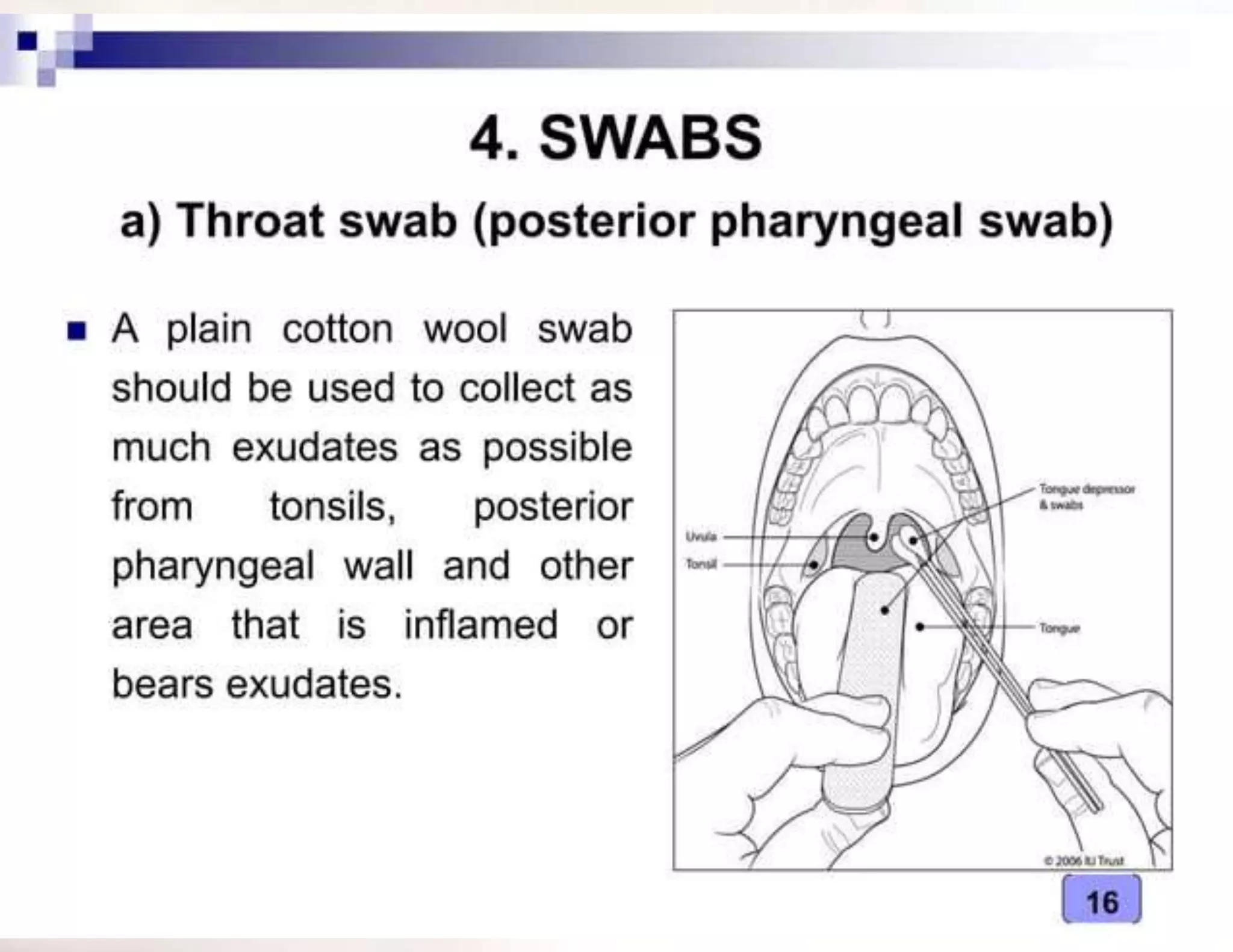
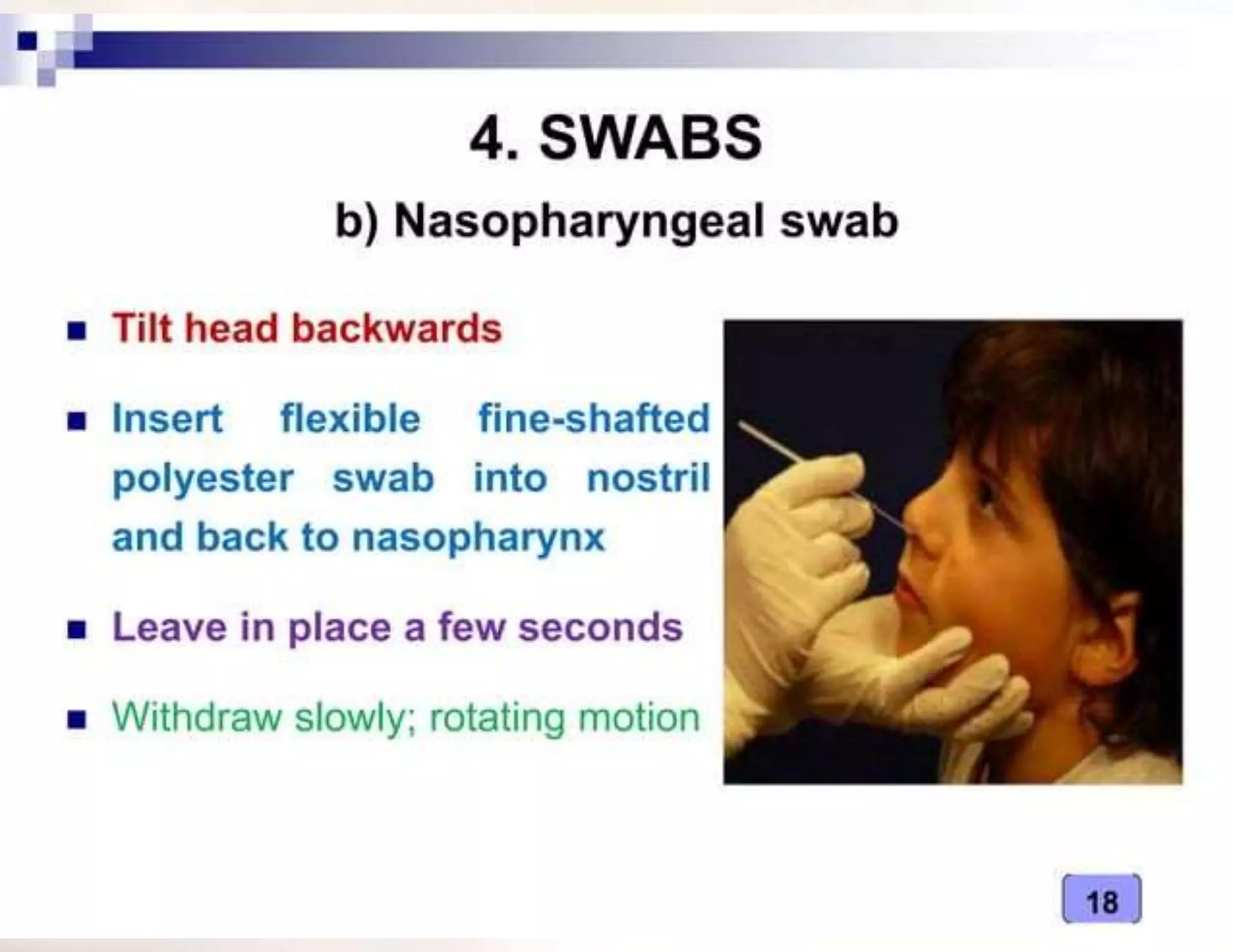
- The type of specimen and collection method depends on the infection or test being performed. Examples provided include blood, urine, stool and sputum collection procedures.
- Transport media is used to maintain specimen viability during transit to the laboratory and varies based on specimen type. Strict