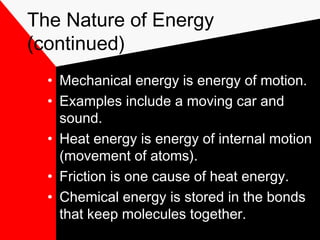
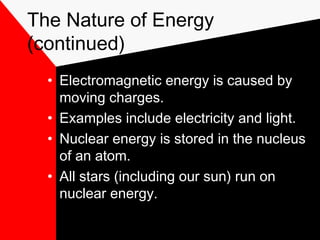
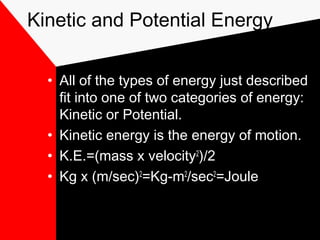
This document discusses different forms of energy including mechanical, heat, chemical, electromagnetic, and nuclear energy. It defines kinetic energy as the energy of motion and potential energy as stored energy. The document explains that energy can be converted from one form to another, but the total amount of energy remains constant due to the law of conservation of energy. Understanding energy and its transformations is important for studying physics and the physical sciences.