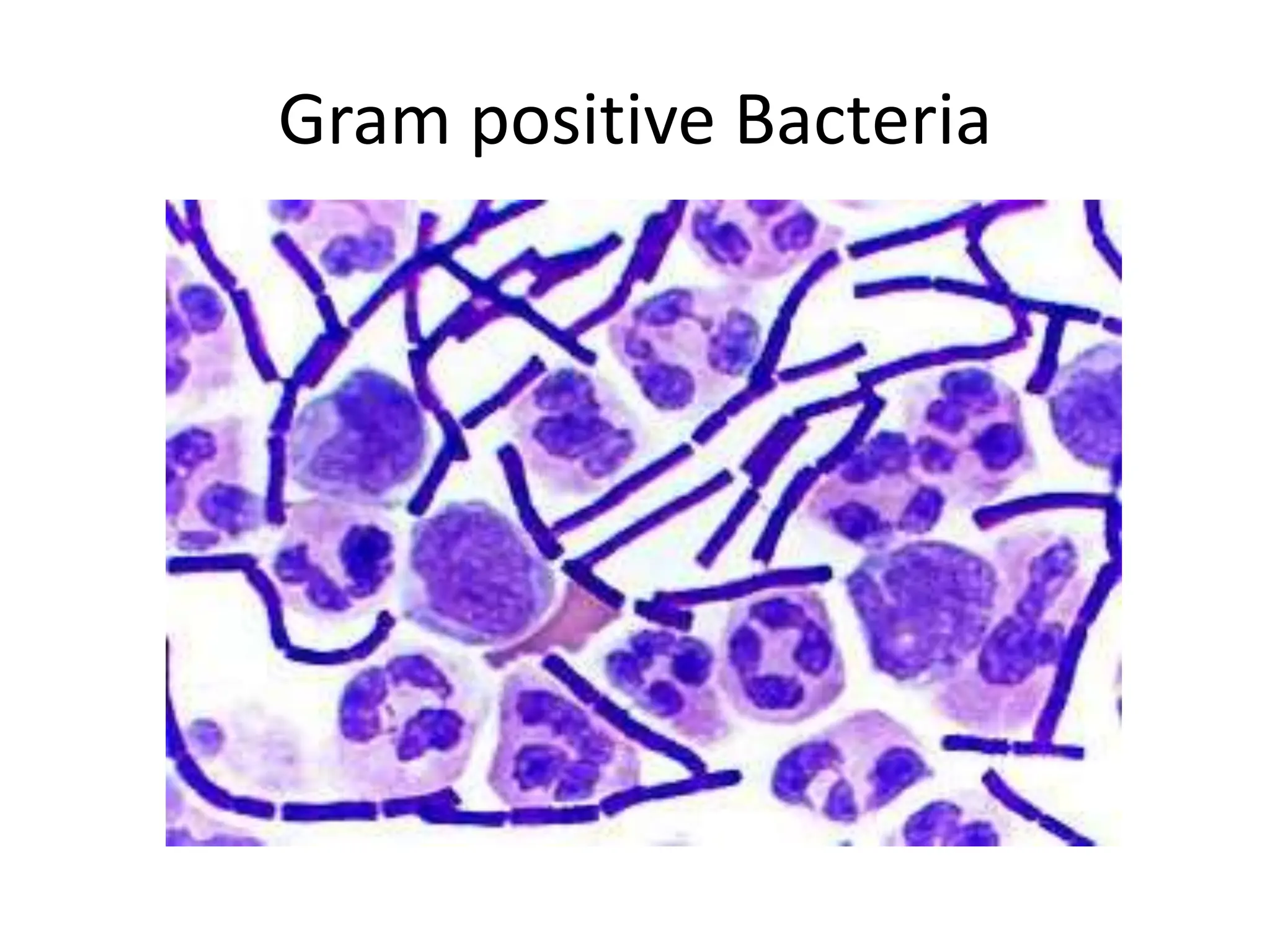
This document discusses diagnostic methods in microbiology. It outlines various laboratory techniques used to diagnose infectious diseases, including microscopy, culture-based methods, and biochemical tests. Specific staining techniques are described, such as Gram stain, acid-fast stain, spore stains, and potassium hydroxide testing. Proper collection, transport, and storage of biological specimens is also emphasized as crucial for obtaining accurate and timely microbiological results.


























![• Transport of Specimens
• Storage of specimens
• Specimens that should not be refrigerated
include:
* blood--should be left at room temperature
or in an incubator at 5[degrees]C
* cerebrospinal fluid--transport at room
temperature
* Neisseria species--transport rapidly to the
laboratory.
Basic Issues in Proper Handling of
Specimens](https://image.slidesharecdn.com/methodsindiagnosticmicrobiologyppt-240312140706-42816058/75/methods-in-diagnostic-microbiology-ppt-pptx-32-2048.jpg)
