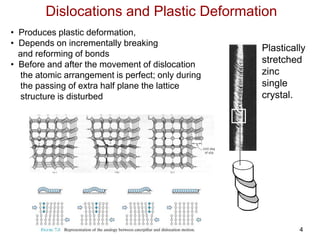
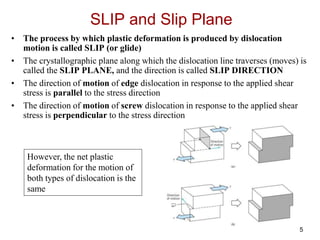
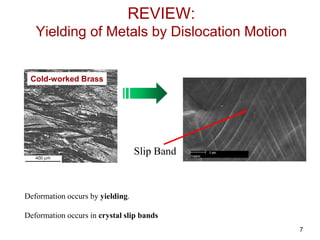
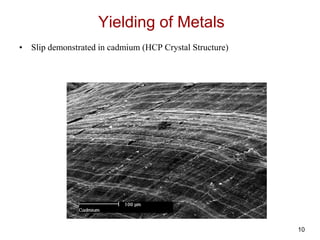
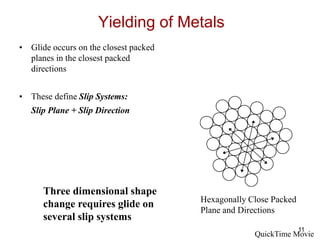
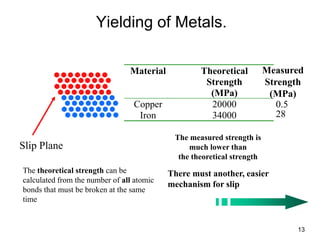
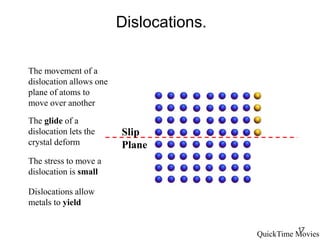
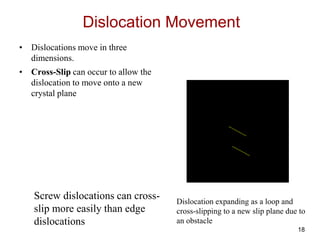
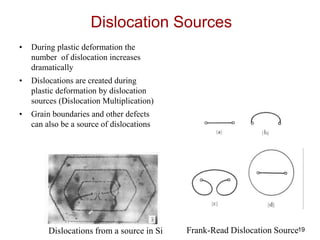
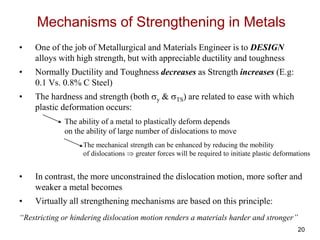
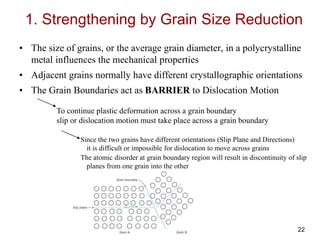
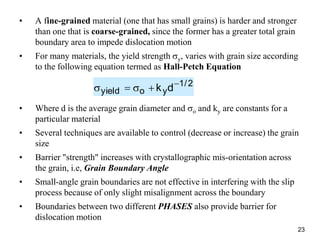
This document discusses strengthening mechanisms in metals by impeding dislocation motion. It introduces four main mechanisms: 1) reducing grain size according to the Hall-Petch relationship, 2) solid solution strengthening through alloying with impurity atoms that distort the lattice, 3) strain hardening as dislocations interact and impede each other with increasing plastic deformation, and 4) dispersion strengthening by particles that obstruct dislocation motion. The key to strengthening metals is restricting dislocation motion to require greater forces for plastic deformation.