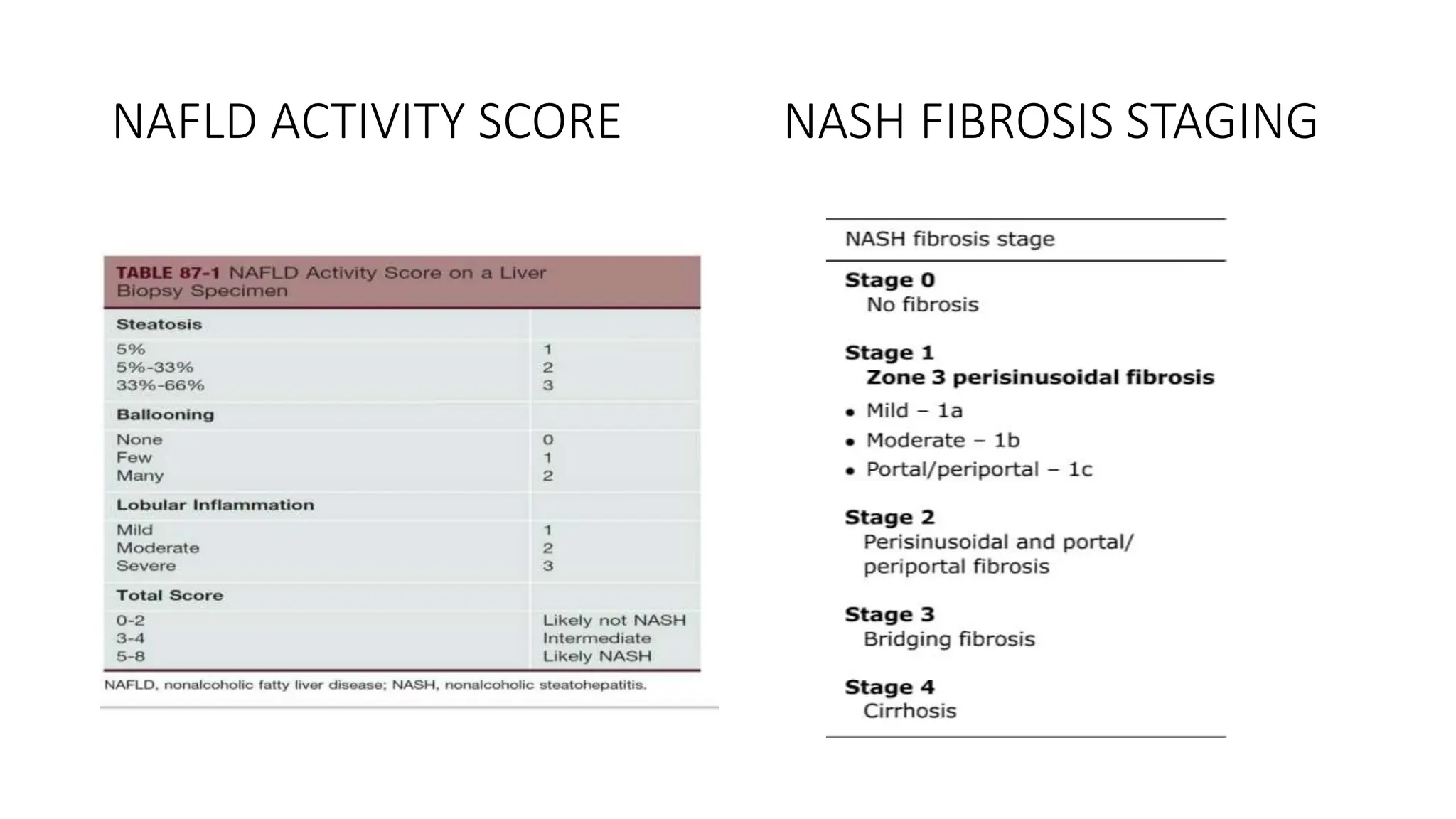
This document provides an overview of non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). It discusses the new nomenclature of metabolic dysfunction associated steatotic liver disease (MASLD) and metabolic dysfunction associated steatohepatitis (MASH). The document reviews the prevalence, risk factors, pathogenesis, clinical features, diagnostic approach and management options for NAFLD/NASH. It provides details on non-invasive and invasive testing methods as well as histological scoring systems used to evaluate NAFLD and NASH.