This document outlines the application process and required documents for various medical device licenses from India's Central Drugs Standard Control Organization (CDSCO). It provides details on:
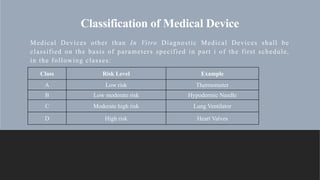
1. Medical device classification based on risk level into Classes A through D.
2. The application process and documents required for an import license, manufacturing license, loan manufacturing license for Classes A and B devices, and manufacturing license for Classes C and D devices.
3. The documents include regulatory certificates, quality management documentation, device master files, and other technical documents describing the device and quality systems.