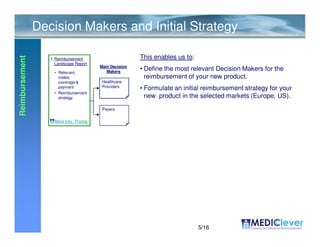
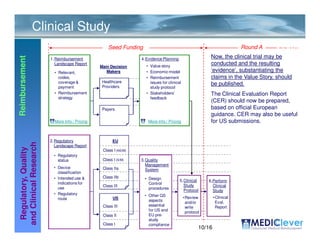
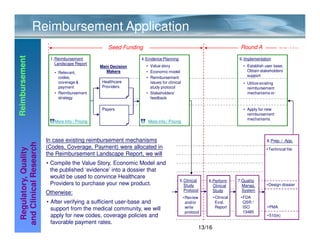
Mediclever Ltd. provides regulatory, quality, reimbursement, and clinical research consulting services to life science start-ups in Europe and the USA. Their services include regulatory landscape reports, quality management system implementation, clinical study planning and execution, reimbursement strategy development, and applications for regulatory approval and reimbursement. They work in collaboration with partners who can coordinate and manage the provision of these services.