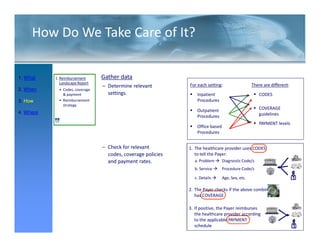
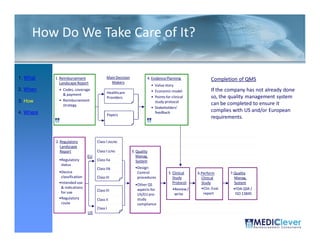
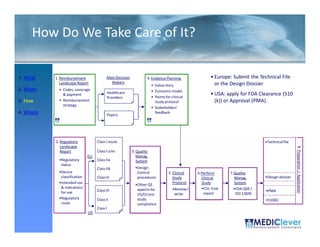
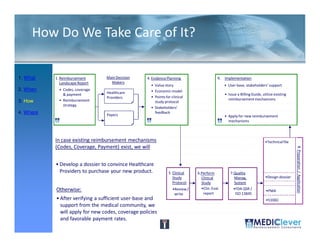
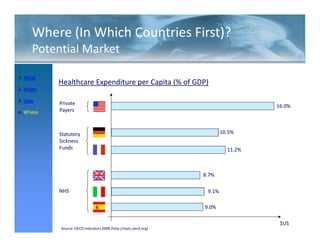
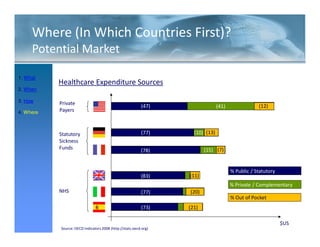
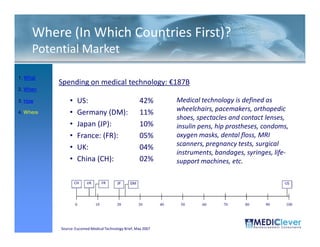
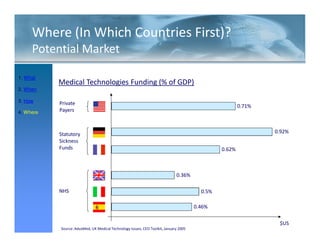
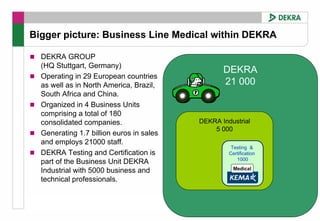
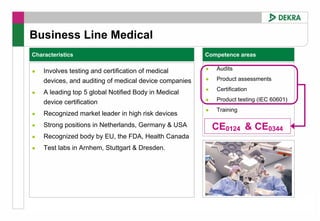
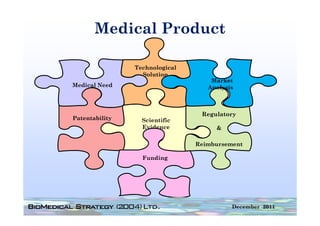
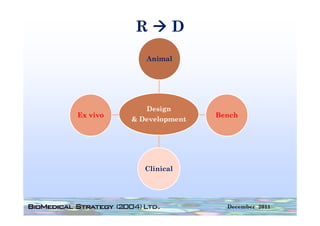
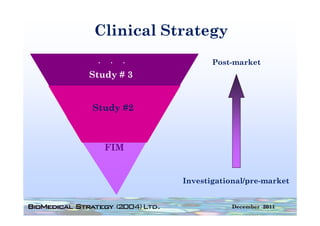
This FDA warning letter outlines issues with a clinical investigation conducted by Dr. Thomas Beilke between 2008-2009. The FDA inspection found that Dr. Beilke failed to properly conduct or supervise the clinical investigation according to regulations. Specifically, the letter cites that Dr. Beilke did not personally conduct or supervise the investigation as required. The FDA concluded that Dr. Beilke did not adhere to statutory requirements and regulations governing clinical investigations.







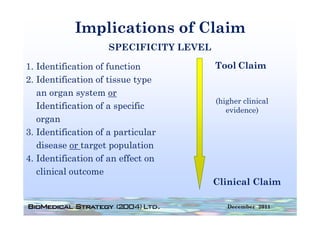



























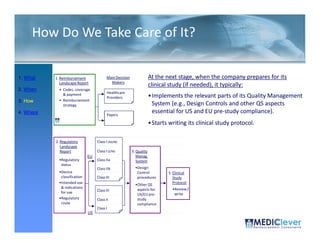
![FDA Warning Letter (1)
Between July 26 and August 24, 2010, Thomas R. Beilke, representing the
U.S. Food and Drug Administration (FDA), conducted an investigation of your
former practice
practice.
During the course of the inspection, Mr. Beilke met with you to
review your conduct of a clinical investigation (Protocol (b)(4), titled
"(b)(4)") performed for (b)(4). You were the investigator for this clinical
investigation between March 2008 and March 2009……..
From our review of the establishment inspection report and
the documents submitted with th t report, we conclude th t
th d t b itt d ith that t l d that
you did not adhere to the applicable statutory requirements
and FDA regulations governing the conduct of clinical
investigations.
1. You failed to ensure that the investigation was conducted
according to the signed investigator statement, in that you failed to
g g g , y
personally conduct or supervise the clinical investigation [21 CFR
312.60].
2. You failed to ensure that the investigation was conducted
acco di g to the investigational plan [21 CFR 312 60]
according i estigatio al la 312.60].
December 2011](https://image.slidesharecdn.com/biomedicalstrategyworkshoppresentations-13236109105882-phpapp02-111211074921-phpapp02/85/BioMedical-Strategy-Medical-Devices-Workshop-Presentations-50-320.jpg)
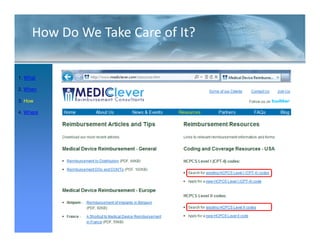
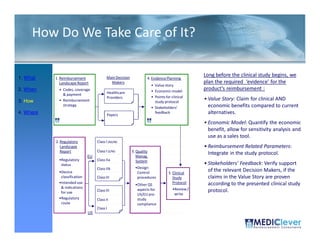
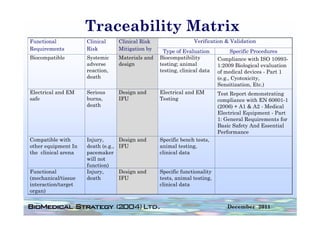
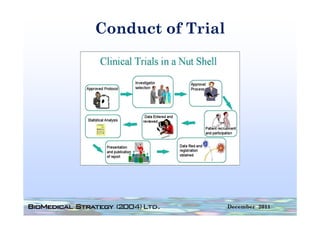
![FDA Warning Letter (2)
This Warning Letter is to inform you of objectionable conditions observed
during the Food and Drug Administration (FDA) inspection conducted at
Orthocon, Inc.
Orthocon Inc ...
The purpose of this inspection was to determine whether activities as
sponsor of the clinical studies (b)(4) and (b)(4) complied with
applicable federal regulations.
This letter also requests prompt corrective action to address the violation
cited and discusses your written response dated September 23 2010 to the
23,
noted violation. Failure to secure the investigator’s compliance. [21
CFR 812.46(a)]: Sponsors are responsible for monitoring and
ensuring compliance of clinical investigators participating in the
investigation.
A sponsor who discovers that an investigator is not complying with the
signed agreement the investigational plan applicable FDA regulations or
agreement, plan, regulations,
any conditions of approval imposed by the reviewing IRB or FDA shall
promptly either secure compliance or discontinue shipments of the device
to the investigator and terminate the investigator’s participation in the
investigation.
December 2011](https://image.slidesharecdn.com/biomedicalstrategyworkshoppresentations-13236109105882-phpapp02-111211074921-phpapp02/85/BioMedical-Strategy-Medical-Devices-Workshop-Presentations-51-320.jpg)





































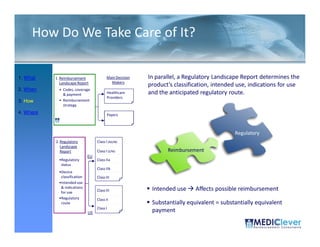
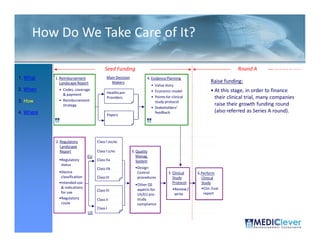
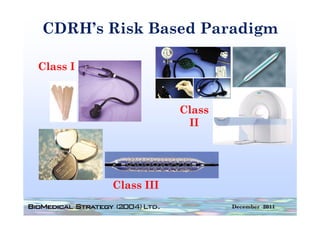
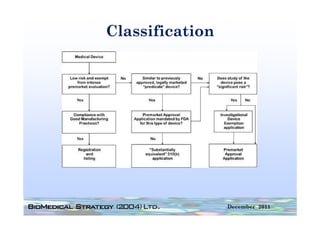
![Principles of Law-VII
• “A device is ‘substantially equivalent’ if it has
the same intended use and the same
technological characteristics as those of the
existing device.
• “Thus, the 510(k) submission compares the
accused product, Baxa's compounders, with
the commercial embodiment of the ‘010
patent, Clintec's compounders. “ [I]t is error for
a court to compare in its infringement analysis
the accused product . . . with the patentee's
commercial embodiment.” …Although in the
recitation of facts, the court in United States
Surgical Corp. v. Hospital Prods. Int'l Pty. Ltd.,
remarked that statements in the Section
510(k) submission “may be construed as
admission of infringement,” the court did not
rely on the submission in the infringement
analysis.”](https://image.slidesharecdn.com/biomedicalstrategyworkshoppresentations-13236109105882-phpapp02-111211074921-phpapp02/85/BioMedical-Strategy-Medical-Devices-Workshop-Presentations-89-320.jpg)