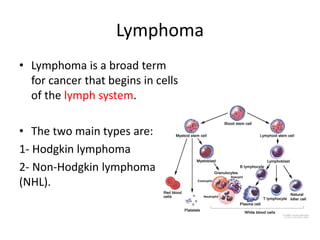
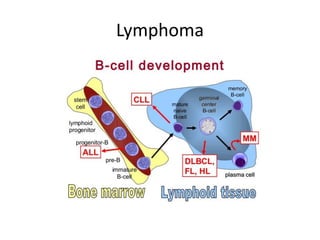
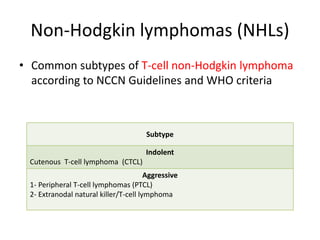
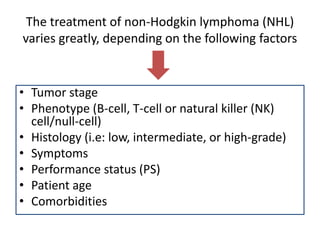
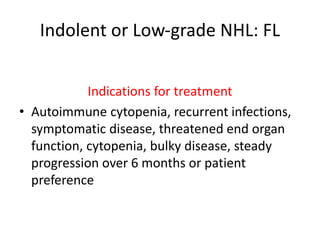
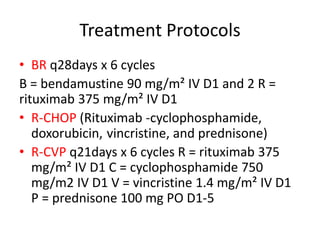
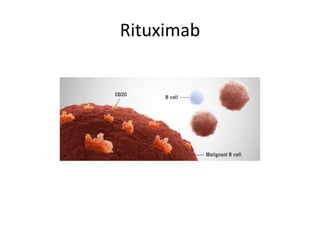
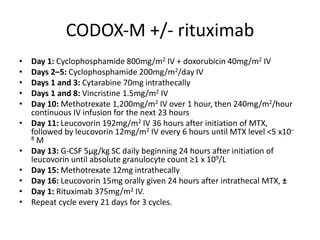
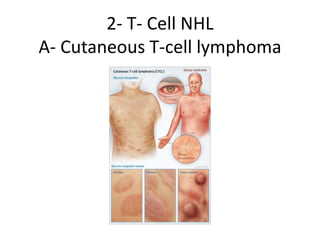
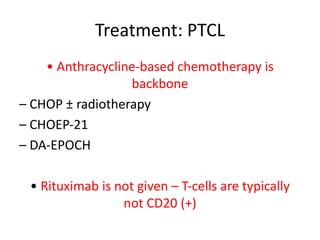
This document provides an overview of Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL). It discusses the definition, epidemiology, risk factors, signs/symptoms, diagnosis, classification, and treatment of HL. It also discusses the overview, epidemiology, etiology, classification, and treatment of several common subtypes of NHL, including follicular lymphoma, diffuse large B-cell lymphoma, and mantle cell lymphoma. Treatment options discussed include chemotherapy regimens like ABVD, R-CHOP, radiotherapy, immunotherapy with rituximab, and newer targeted therapies.