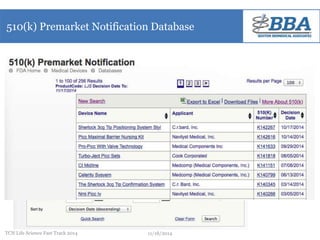
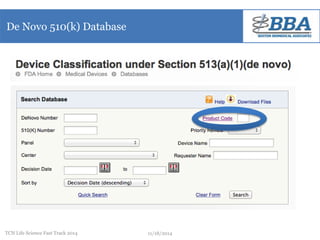
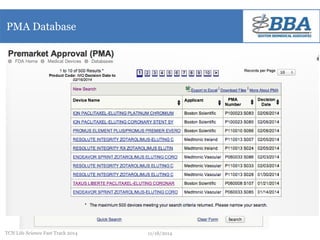
This document outlines a regulatory strategy for product approval within the life sciences sector, emphasizing the importance of understanding regulatory pathways, product classifications, and FDA engagement for successful market entry. It details the steps for pre-market regulatory planning including product categorization, determining appropriate submission types (PMA or 510(k)), and conducting market research. The document also highlights the need for effective regulatory discussions and preparation strategies when interacting with the FDA.