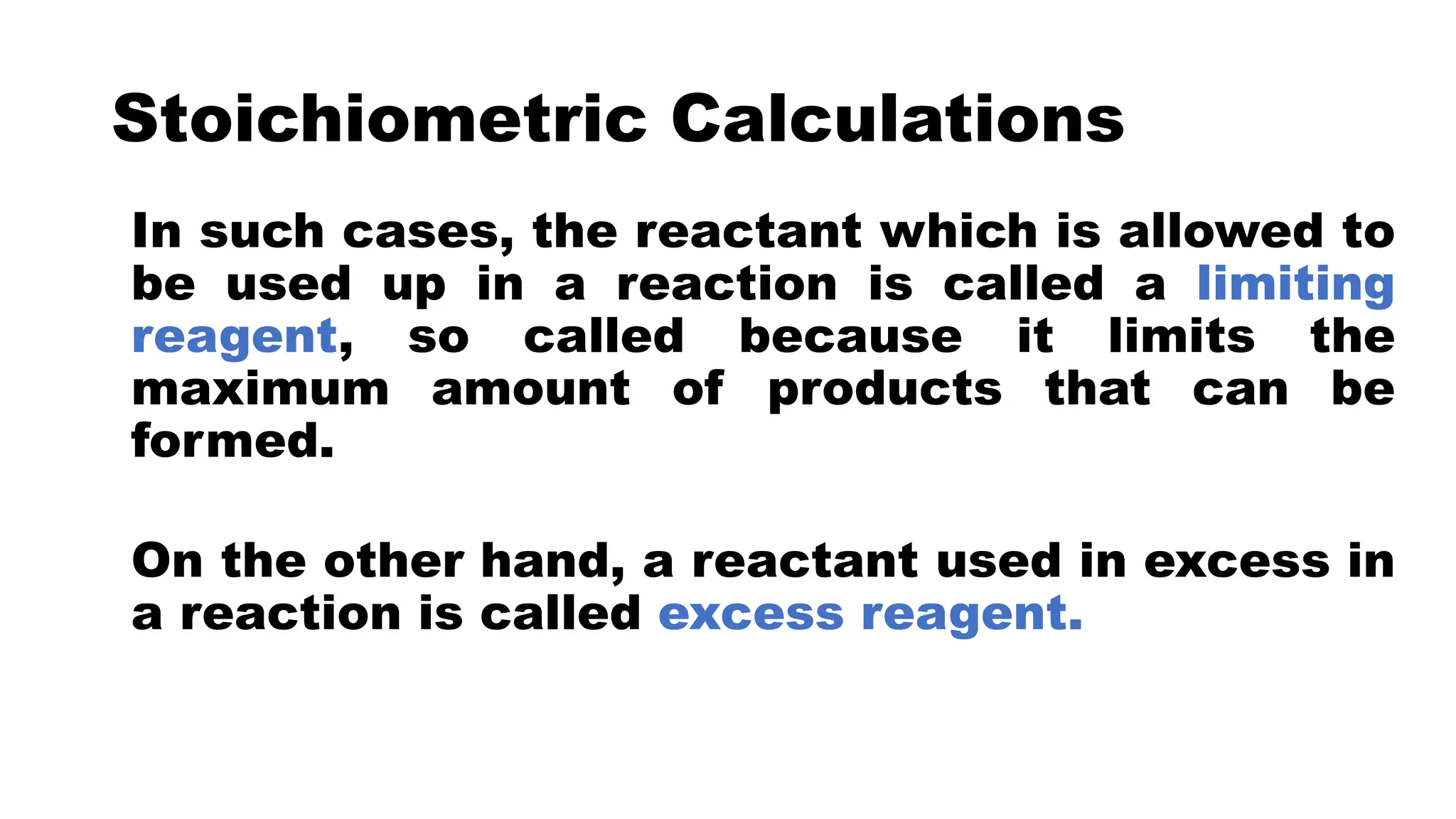
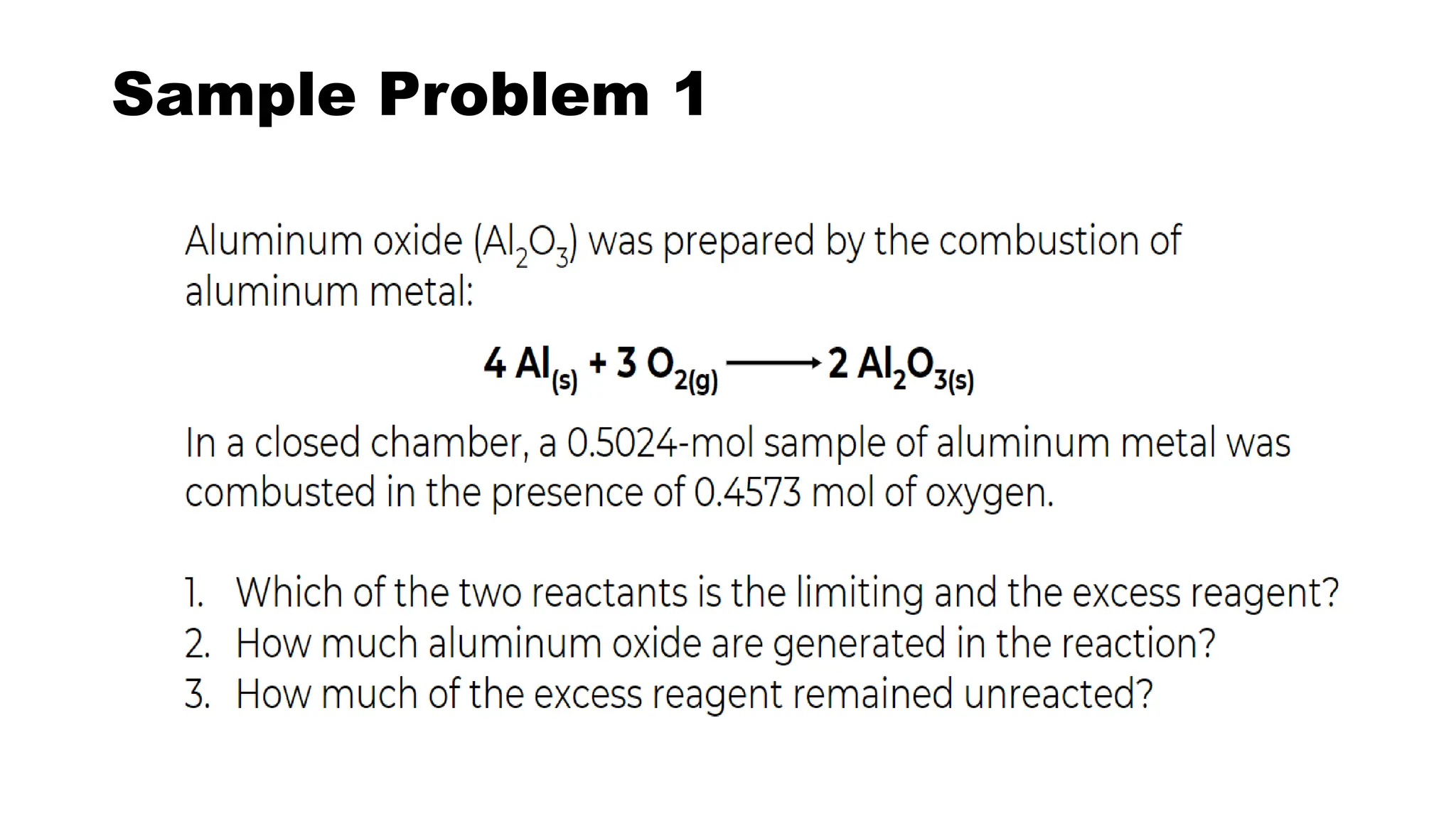
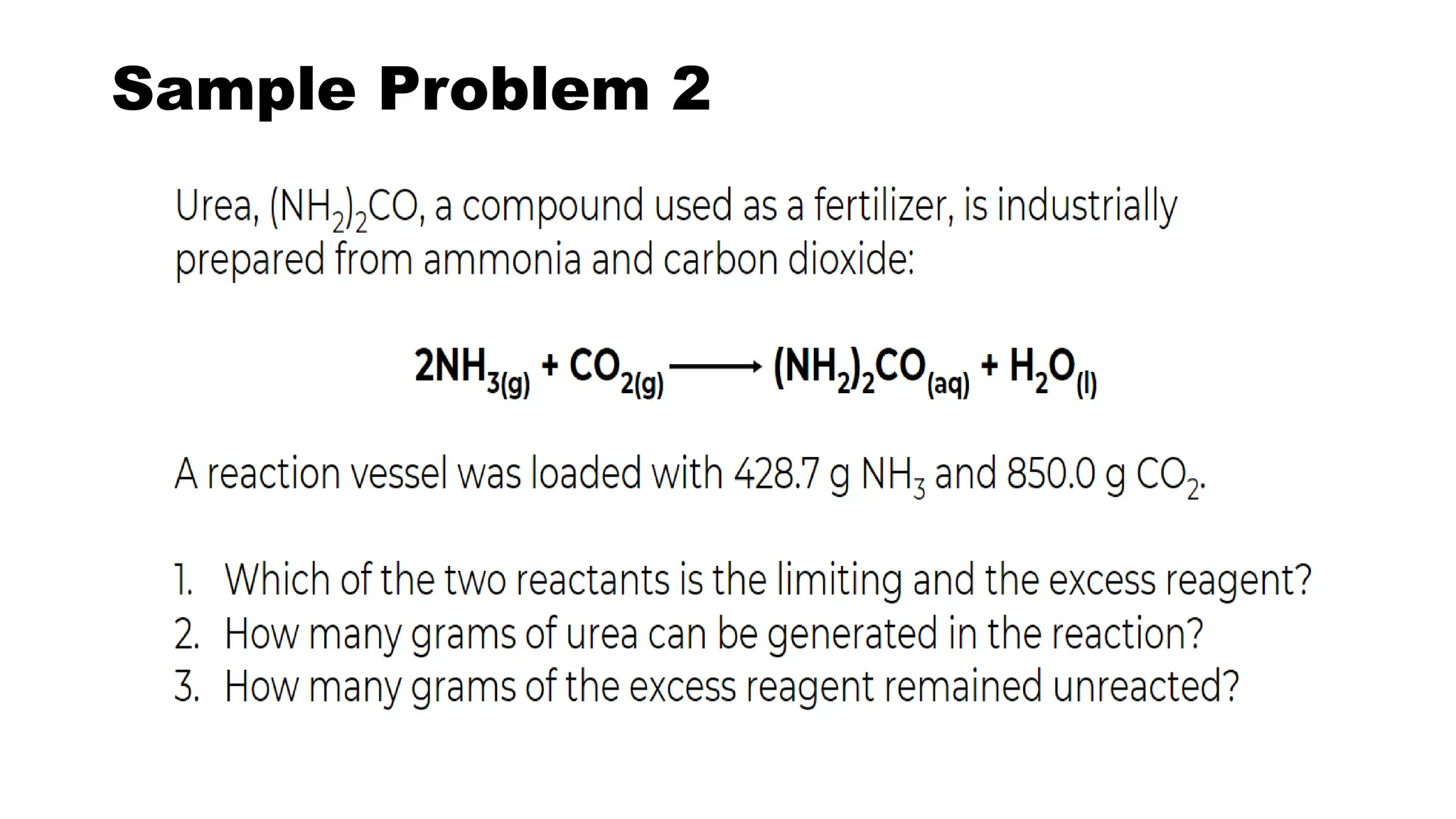
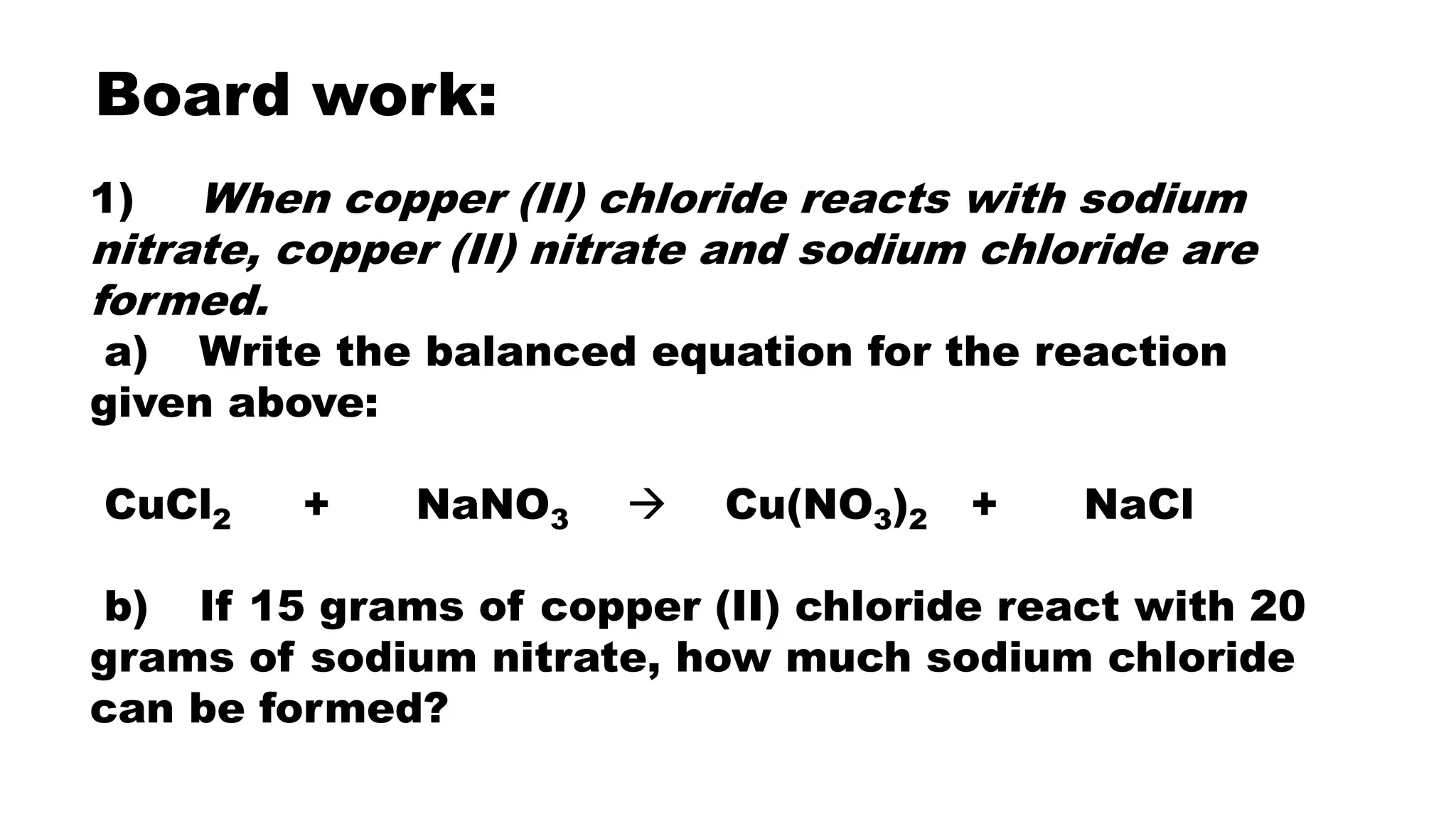
The document discusses limiting and excess reactants in stoichiometric calculations. A limiting reactant is the reactant that is used up first in a chemical reaction and limits the amount of products that can be formed. Any reactant in excess not used up in the reaction is called the excess reactant. Sample problems are provided to demonstrate how to identify the limiting and excess reactants and use stoichiometric calculations to determine the maximum amount of products and leftover excess reactant.