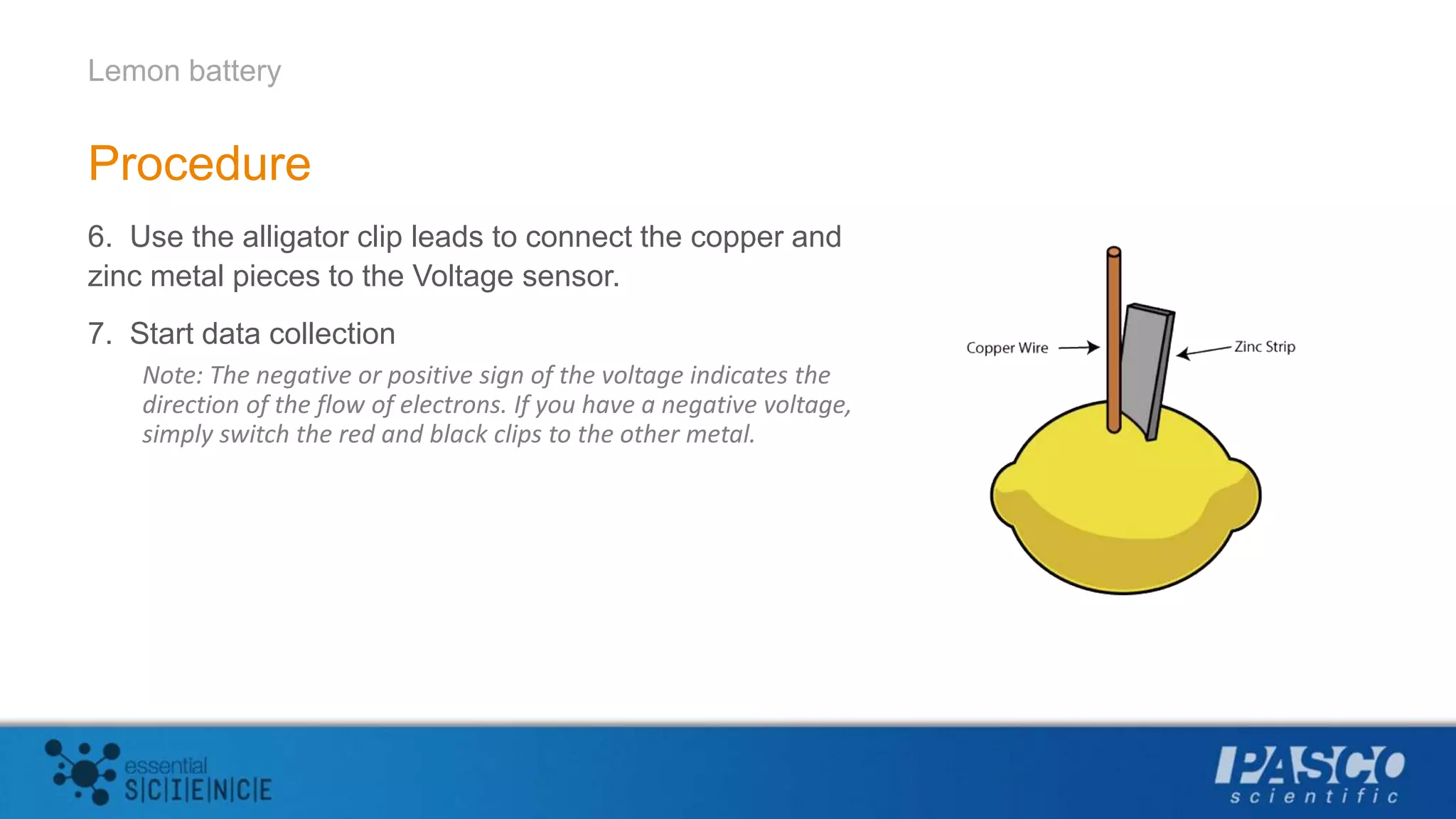
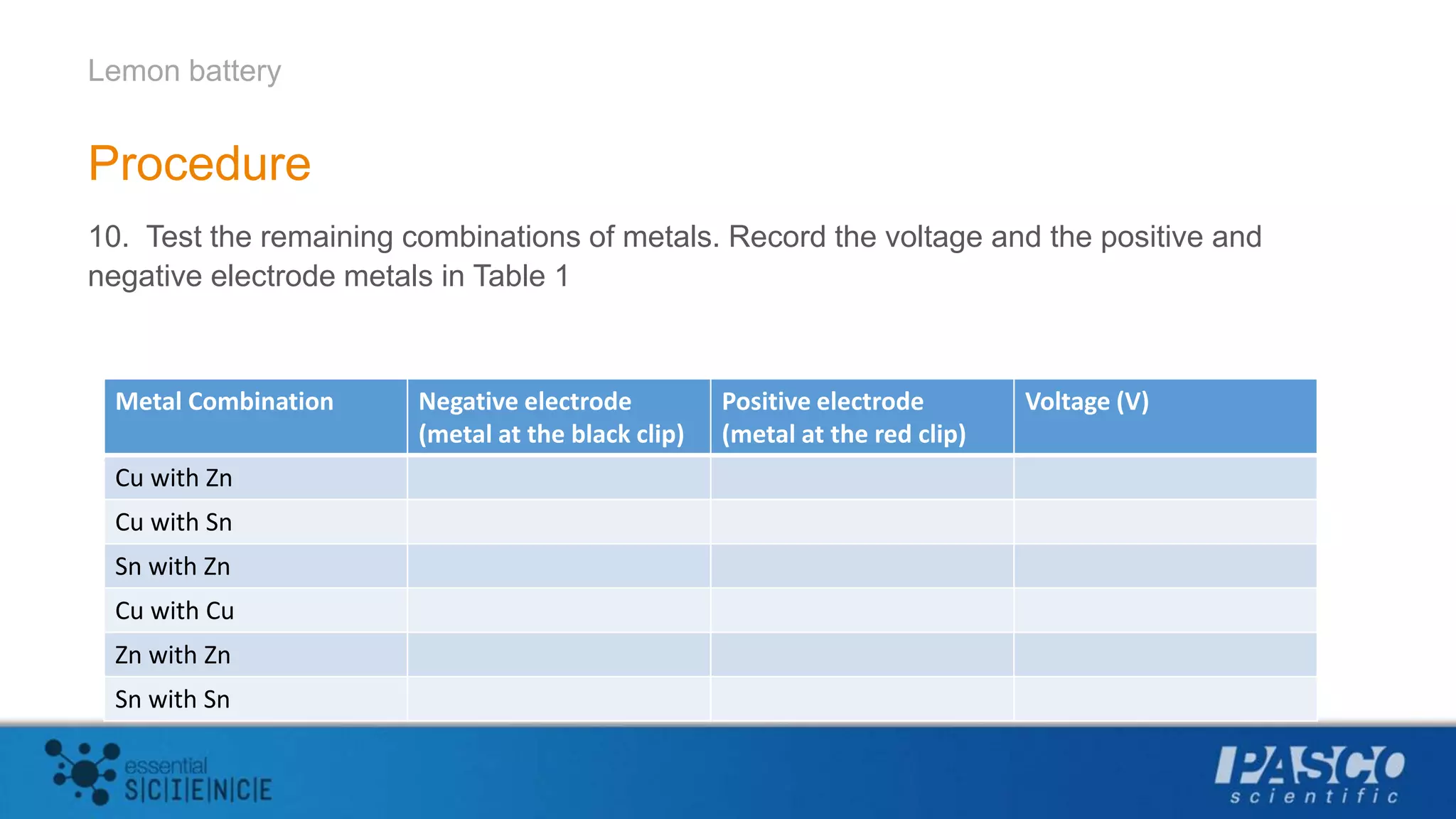
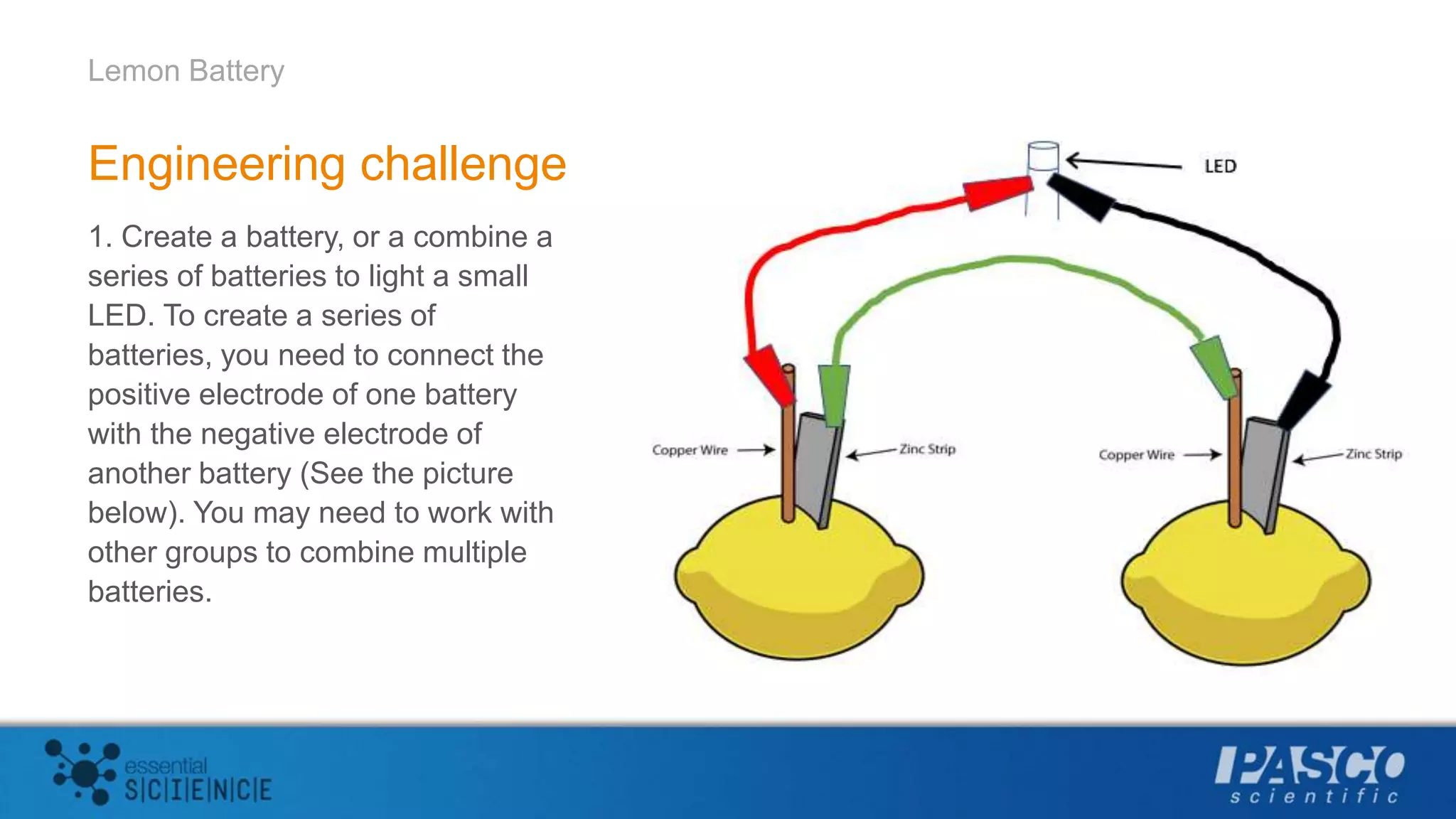
This document describes an experiment using lemons to create a battery. Students insert zinc and copper strips into a lemon and connect them to a voltage sensor to measure the voltage generated. Combining zinc and copper produced the largest voltage of around 1 volt. The experiment aims to demonstrate that batteries generate electricity through redox reactions, with metals like zinc losing electrons and metals like copper gaining electrons. Multiple lemon batteries can be connected in series to generate enough voltage to light an LED.