The document outlines the legislation relating to food safety and biosafety in Nigeria, emphasizing the historical development of laws aimed at ensuring the safety and wholesomeness of food products. It discusses the role of various legal bodies, including NAFDAC, in regulating food safety and details the provisions of the Nigerian Biosafety Act of 2015, which governs the importation and use of genetically modified organisms. Additionally, it highlights the importance of mycotoxin regulations and the need for a coordinated food safety policy to protect consumers and promote economic interests.





.
6](https://image.slidesharecdn.com/legislationrelatingtodevelopedproducts-180425212057/85/Legislation-relating-to-developed-products-6-320.jpg)

















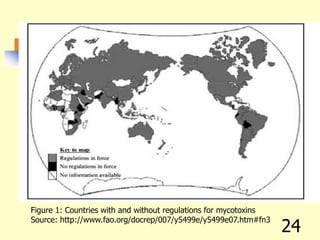
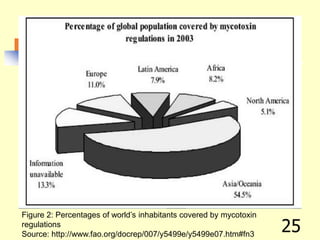
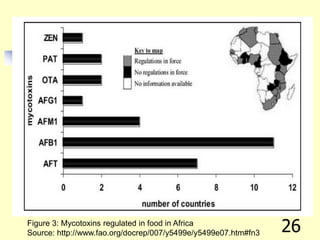
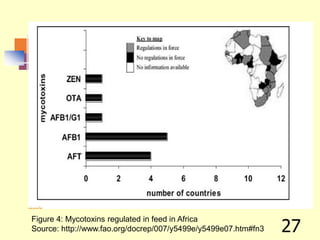

![REFERENCES
Jane Omojokun (April 10th 2013). Regulation and Enforcement of Legislation on
Food Safety in Nigeria, Mycotoxin and Food Safety in Developing Countries Hussaini
Makun, IntechOpen, DOI: 10.5772/54423. Available from:
https://www.intechopen.com/books/mycotoxin-and-food-safety-in-developing-
countries/regulation-and-enforcement-of-legislation-on-food-safety-in-nigeria
Arvanitoyannis, I. S., Choreftaki, S., & Tserkezou, P. (2006). Presentation and
comments on EU legislation related to food industries–environment interactions:
sustainable development, and protection of nature and biodiversity – genetically
modified organisms. International Journal of Food Science & Technology, 41(7):
813-832. doi: doi:10.1111/j.1365-2621.2006.01252.x
BRINK, Johan A; WOODWARD, Barbara R y DASILVA, Edgar J. Plant biotechnology:
a tool for development in Africa. Electron. J. Biotechnol. [online]. 1998, vol.1, n.3,
pp.14-15. ISSN 0717-3458. http://dx.doi.org/10.4067/S0717-34581998000300004.
https://www.vanguardngr.com/2015/04/nigeria-gets-biosafety-law-joins-league-of-
biotech-countries/
29](https://image.slidesharecdn.com/legislationrelatingtodevelopedproducts-180425212057/85/Legislation-relating-to-developed-products-29-320.jpg)
