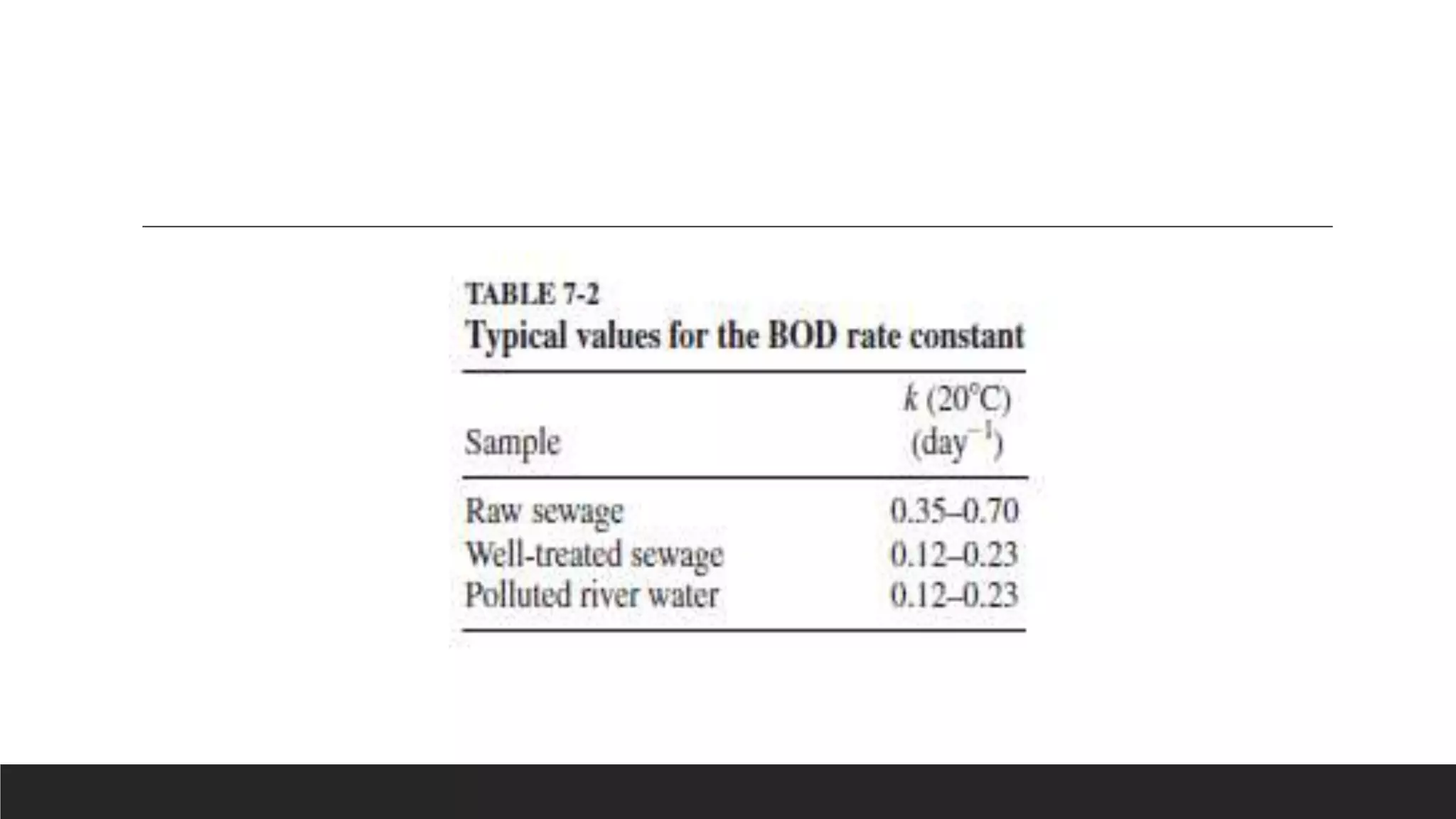
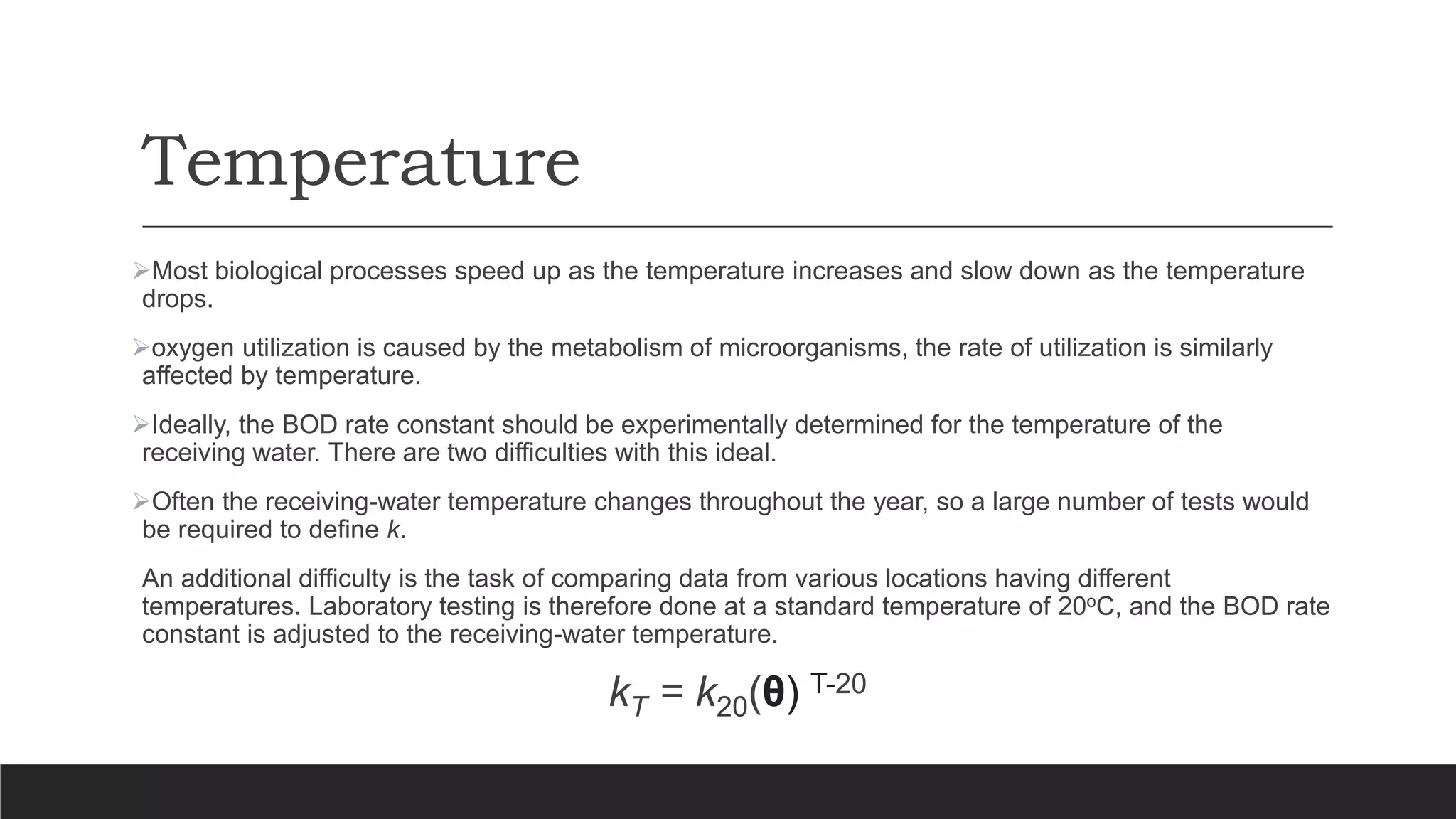
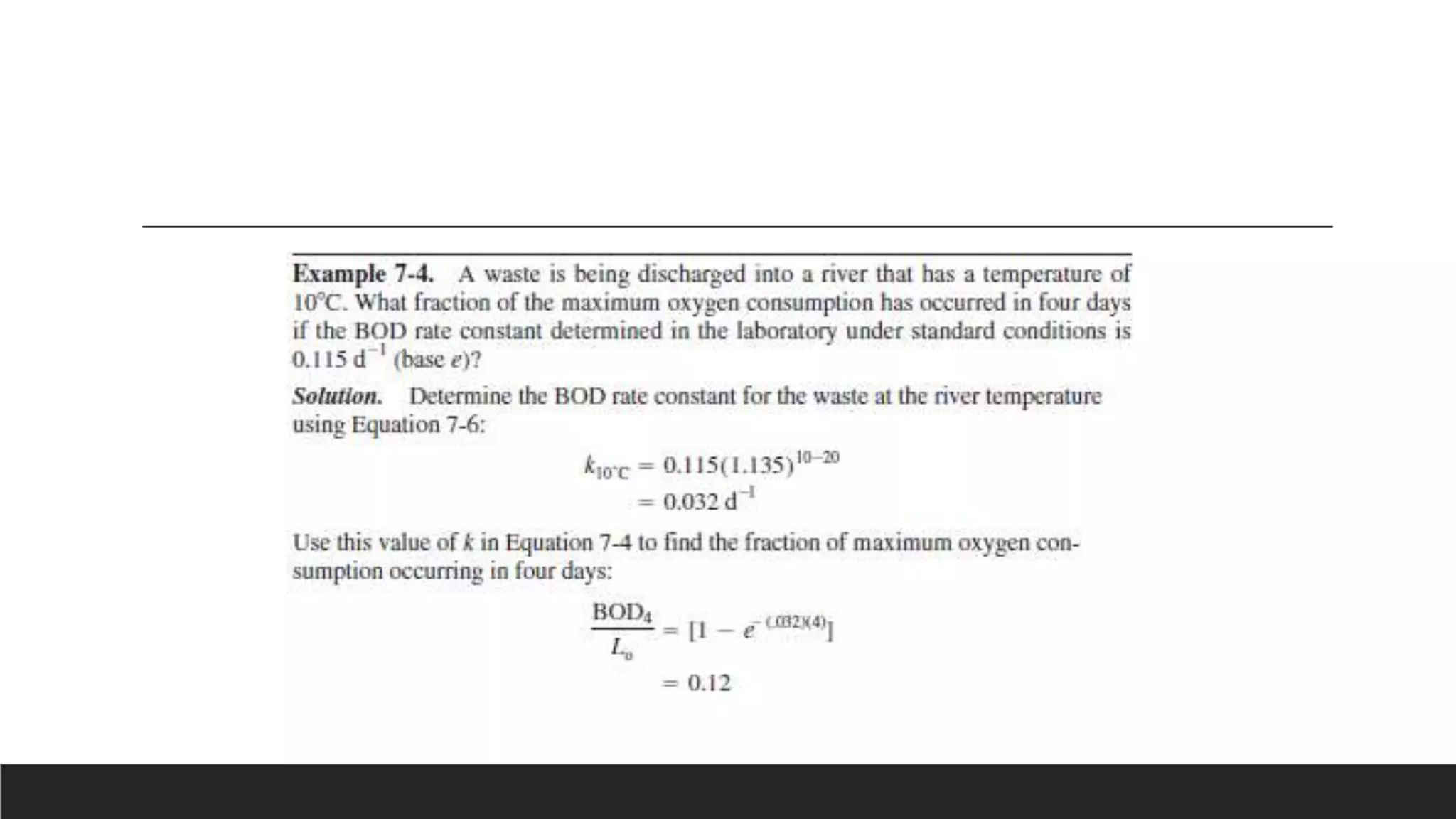
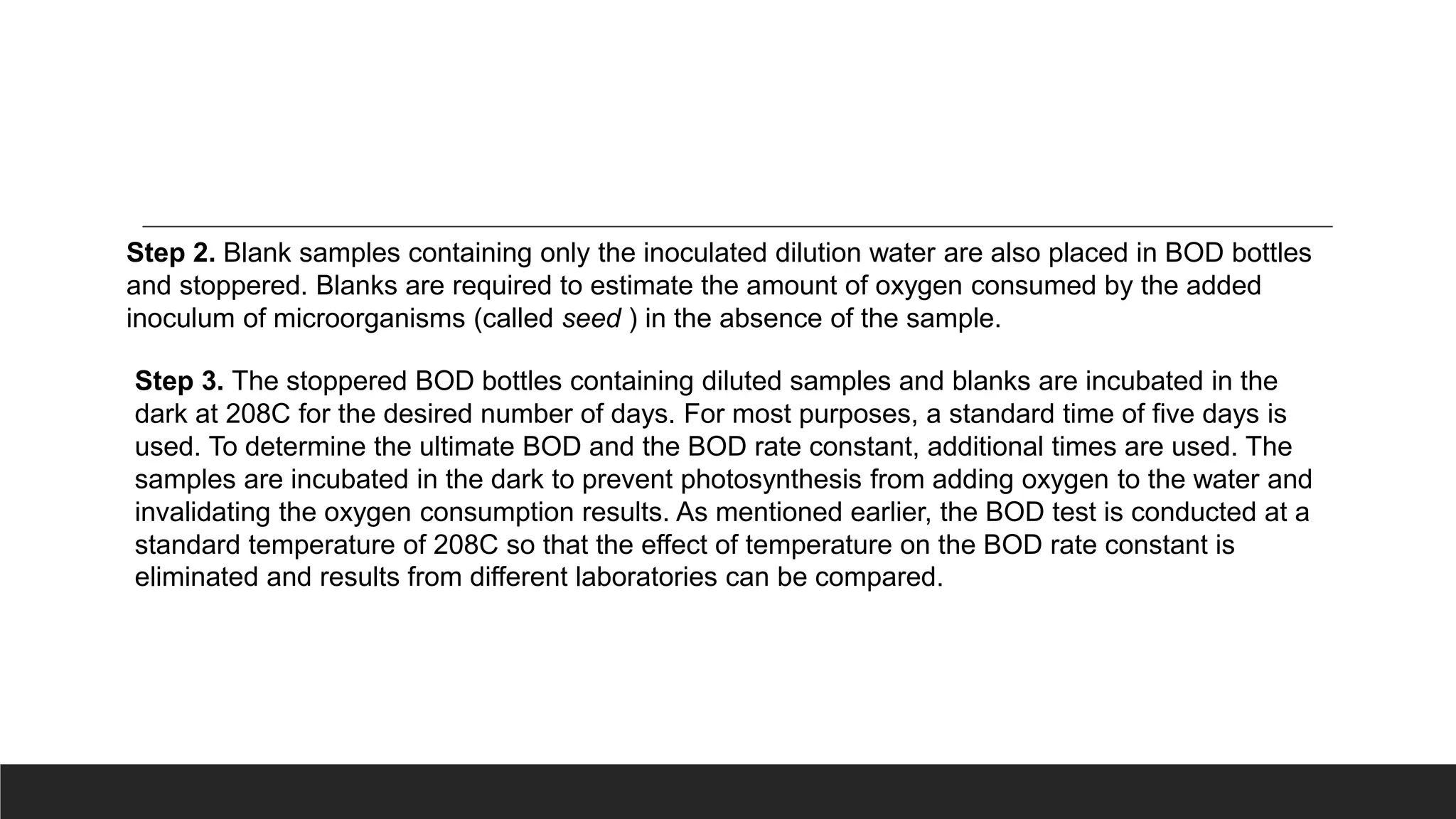
This document discusses factors that affect biochemical oxygen demand (BOD), including ultimate BOD, BOD rate constant, nature of waste, ability of organisms to utilize waste, and temperature. Ultimate BOD represents the maximum oxygen demand of a waste. The BOD rate constant depends on these factors and indicates how quickly oxygen will be depleted. Simple sugars degrade quickly while more complex compounds degrade more slowly. The organisms used to inoculate BOD tests may not be able to degrade all waste components. BOD tests are conducted at 20°C to standardize temperature effects and allow comparison of results.