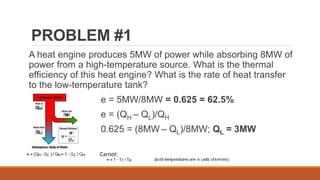
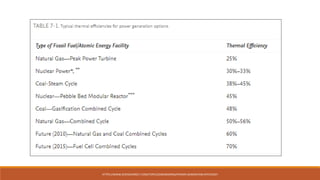
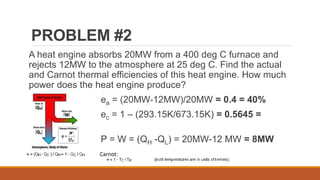
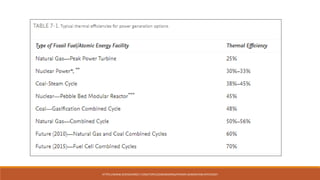
The document discusses violations of the laws of thermodynamics through perpetual motion machines. It defines the three laws of thermodynamics and explains that perpetual motion machines would violate the first and/or second law. There are three types of perpetual motion machines described: type 1 produces work without energy input, type 2 converts heat spontaneously to work without cooling a reservoir, and type 3 aims to eliminate all friction to maintain motion indefinitely through inertia. The document also provides examples of problems calculating thermal efficiencies of heat engines.