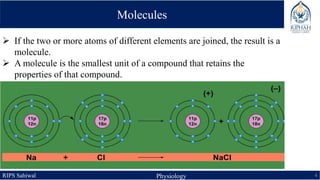
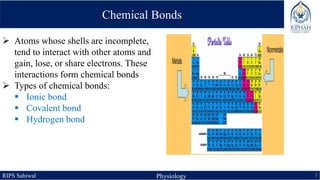
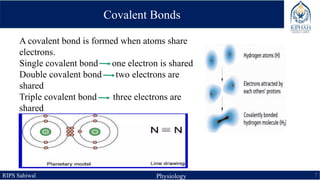
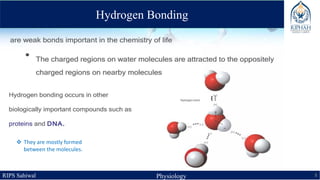
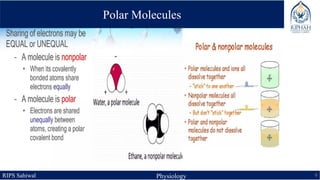
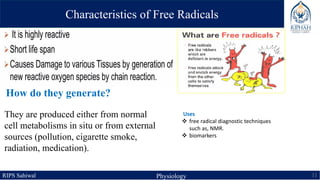
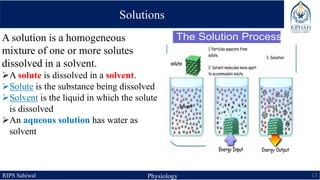
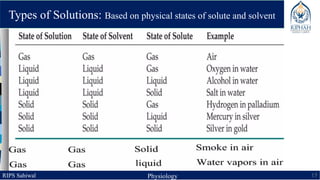
This document provides an overview of chemical composition of the body. It discusses molecules and chemical bonds including ionic bonds, covalent bonds, and hydrogen bonds. It also describes polar molecules, free radicals, and solutions. Finally, it explains the importance of water as a universal solvent in the body and its unique properties including hydrogen bonding that allow it to regulate temperature and dissolve other substances.