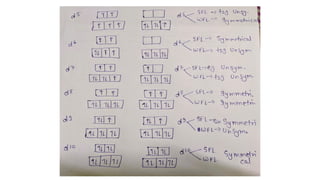
The John-Teller effect is a geometric distortion observed in octahedral complexes, reducing their symmetry and energy, particularly when d-electron configurations lead to degeneracy. This effect can result in either elongation or compression of the complex depending on how the degeneracy is resolved, affecting the energy of specific d orbitals. Notably, this distortion is prevalent in metal complexes like copper(II), while certain configurations do not exhibit this phenomenon.


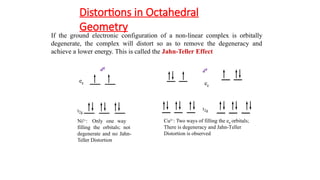
![John-Teller Distortion in Cu(II)
Complexes
dx2-y2
eg
energy
dz2
dxy
t2g
Cu(II) in regular octa-
hedral environment
dxz dyz
Cu(II) after John teller
distortion
6
[CuF ]4-](https://image.slidesharecdn.com/2f3tamplsvavhw9hd9or-john-teller-241129063945-d0e76cb3/85/John_Teller-effect-in-octahedral-complexs-pptx-9-320.jpg)




