This document discusses a density functional theory study of the epimerization of glucose to mannose catalyzed by Sn-beta zeolite and borate salts. The study finds that the tetrahedral borate ion forms a complex with glucose that inhibits competitive isomerization. The stannanol group of Sn-beta catalyzes glucose ring opening, followed by enolization catalyzed by the silanol group. Epimerization then proceeds via an intramolecular 1,2 carbon shift, which is the rate-limiting step. Tin is found to be the most active metal center, and the proximity of the silanol group to the stannanol group enhances catalytic activity. The study aims to understand the cooperative roles of
![Brønsted and Lewis acid sites of Sn-beta zeolite, in combination
with the borate salt, catalyze the epimerization
of glucose: A density functional theory study
B.K. Chethana, Samir H. Mushrif ⇑
School of Chemical and Biomedical Engineering, Nanyang Technological University, 62 Nanyang Drive, Singapore 637459, Singapore
a r t i c l e i n f o
Article history:
Received 22 September 2014
Revised 20 November 2014
Accepted 6 January 2015
Keywords:
Glucose epimerization
Sn-beta zeolite
Borate salt
Density functional theory
Mannose
Bilik mechanism
a b s t r a c t
Sn-beta zeolite, in combination with borate salts, is a potential inorganic catalyst for sugars epimeriza-
tion. We investigate, at molecular level, the catalytic mechanism of glucose epimerization to mannose,
using density functional theory. Our calculations suggest that the tetrahedral borate ion forms a complex
with glucose and inhibits the competitive isomerization reaction. The Lewis-acidic stannanol group of Sn-
beta catalyzes glucose ring opening, which is followed by the silanol group (Brønsted acid site) catalyzed
enolization. The epimerization then proceeds via an intramolecular 1,2 carbon shift and is found to be the
rate-limiting step with an activation enthalpy of 26.3 kcal/mol. Catalytic activities of different tetravalent
metal centers are compared, and Sn is found to be the most active metal. Additionally, it was found that
the proximity of silanol group to the stannanol group, within the zeolitic framework, plays a key role in
enhancing the catalytic activity of the silanol group. Hence, it is crucial to perform calculations with the
entire ring structure of Sn-beta that opens up due to the hydrolysis of Sn–O–Si bridge.
Ó 2015 Elsevier Inc. All rights reserved.
1. Introduction
Carbohydrates play an essential role in food industry, biology,
medicine, and alternate fuel industries [1]. However, out of the
34 pentoses and hexoses, only 7 (D-glucose, D-galactose, D-man-
nose, D-fructose, D-xylose, L-arabinose, and D-ribose) are present
in nature in significant amounts, and the rest are termed as ‘‘rare
sugars.’’ These sugars, though rare, have potential applications in
food and pharmaceutical industries [2–4]. To quote a few, D-taga-
tose is a potential drug for the treatment of Type 2 diabetes [5,6],
xylitol can prevent tooth decay [7], L-ribose is used to prepare a
drug having anti-hepatitis-B virus activity [8], and D-arabinose is
used to prepare antitumor compounds [9,10]. Furthermore, rare
sugars such as xylitol and D-tagatose [11,12] can be substitutes
for existing sugars, since they have comparable sweetness and
have less calorific values. To meet the demand for rare sugars,
expensive and complex biochemical catalysts (such as keto–aldol
isomerases, epimerases, and oxido-reductases) are used to convert
more abundant forms of sugars into rare sugars [13–15]. Despite of
their good performance, enzymatic catalysts face the following
challenges in large-scale processing: (a) requirement of extremely
high purity of the reactant, (b) maintenance of pH within a narrow
(basic) window, (c) constraints on reaction’s temperature, prefera-
bly around 335 K, and (d) regeneration of enzymes is a daunting
task [16]. Therefore, development of simple and more efficient
inorganic catalysts for the production of rare sugars is important.
Unlike enzymatic processes, inorganic catalysts can operate
effectively over a wide range of reactant purities, temperatures,
and pH, and with significant regeneration and separation efficien-
cies. They can reduce the processing cost to a great extent. How-
ever, only a handful of efficient and selective homogeneous and
heterogeneous catalysts have been reported till date, especially
for the epimerization of sugars [17–20]. In an extensive series of
reports, Bilik and co-workers [19,21–23] have shown that C2-epi-
merization of aldose sugars can be catalyzed by Molybdate in mild
acidic conditions. Tanase et al. [24] demonstrated that, the C2 epi-
merization of glucose can also be catalyzed by Ni (II) diamine com-
plexes by stereospecific intramolecular 1,2 carbon shift.
Recently, heterogeneous, solid Lewis acid catalysts have gained
importance in sugars processing due to their unique catalytic activ-
ity, combined with excellent stability in a wide pH and tempera-
ture range [25]. These Lewis acid metal centered catalysts are
capable of activating the carbonyl functional group in sugars in
an aqueous environment. Corma and co-workers [26–29] have
shown that Sn-beta zeolites are active in Bayer–Villiger oxidation
of ketones and Meerwin–Ponndorf–Verely (MPV) reduction of
ketones and aldehydes. Davis and co-workers [30] carried out a
http://dx.doi.org/10.1016/j.jcat.2015.01.008
0021-9517/Ó 2015 Elsevier Inc. All rights reserved.
⇑ Corresponding author. Fax: +65 6794 7553.
E-mail address: SHMushrif@ntu.edu.sg (S.H. Mushrif).
Journal of Catalysis 323 (2015) 158–164
Contents lists available at ScienceDirect
Journal of Catalysis
journal homepage: www.elsevier.com/locate/jcat](https://image.slidesharecdn.com/f6c6b876-32da-4622-9e91-c2803c582c43-150326220923-conversion-gate01/75/J-Catalysis-1-2048.jpg)
![combined experimental and computational study to understand
the catalytic role of Sn-beta zeolite in the glucose isomerization
reaction. In order to leverage on these recent discoveries to design
efficient inorganic catalysts for rare sugars production, Roman–
Leshkov and co-workers [31] demonstrated that Sn-beta zeolites,
when used in combination with sodium borate salt, selectively per-
form epimerization of sugars, thus providing a pathway for the for-
mation of rare sugars using heterogeneous inorganic catalysts.
Although the hydrolyzed Sn-beta center (Fig. 1) is thought to be
the active site [32–35], the catalytic performance of zeolites also
depends on the strength of the acidic silanol group which is adja-
cent to the Lewis acidic metal center [33]. The reported IR spectra
of beta-zeolites show a clearly distinguishable signal of strongly
acidic silanol hydroxyl group at 3740 cmÀ1
[35].
Recently, Vlachos and coworkers [36] also carried out computa-
tional studies to suggest the involvement of silanol group in the
isomerization and epimerization of glucose. They observed that
isomerization is preferred over epimerization, since the activation
energy barrier for isomerization is lower. However, on the con-
trary, experimental studies suggest that, upon the addition of
borate, epimerization is preferred [31]. This demands an investiga-
tion into the Sn-beta-borate combined catalysis in sugars epimer-
ization, to better understand the role of borate in epimerization
and its interference with Sn-beta catalyzed isomerization.
In the present paper, we investigate glucose epimerization, cat-
alyzed by Sn-beta zeolite and borate salts using density functional
theory (DFT). The objectives of the present work are (i) to reveal, at
molecular level, the role of borate salts in promoting the epimer-
ization reaction, (ii) to identify the minimum energy pathway for
epimerization, and (iii) to investigate the possibility of direct par-
ticipation of the silanol group in the keto-enol transformation of
glucose, leading to its epimerization. Understanding the coopera-
tive interaction among the two catalysts and the sugar molecule
will help develop novel inorganic catalysts with high product spec-
ificity and selectivity.
2. Computational methods
Computations in the present work were performed using DFT,
with the B3LYP [37–39] functional. All the calculations were per-
formed using Gaussian-09 suite [40]. Two types of Sn-beta cluster
models have been considered for the calculations (Fig. 1): (i) a
smaller cluster model (T4) consisting of a tetrahedrally coordi-
nated Sn, where there is little or no interaction between the Sn–
OH stannanol and Si–OH silanol groups; and (ii) a larger cluster
model consisting (T9–39 atoms) of the entire ring in the zeolite
framework, where the stannanol and silanol groups are adjacent
to each other. We have carefully constructed the active site of
the larger cluster model by preserving the structural proximity of
silanol and stannanol group. We understand that a cluster model
may have limitations in modeling the long range effects of the
actual solid; however, recently, Vlachos and co-workers [36] have
shown that the size of the cluster does not significantly affect the
energetics of the system as long as we maintain the structural
integrity of silanol and stannanol groups. Similarly, Bell and co-
workers [41] reported isomerization of glucose catalyzed by the
open site of Sn-beta using a larger cluster consisting of 208 atoms
(active site-treated quantum mechanically and rest using molecu-
lar mechanics), and they showed that obtained activation barrier
for the rate-limiting step is in good agreement with the experi-
mental reports [30].
For the present studies, Lewis acid metal center is treated using
LANL2DZ effective core potential basis set recommended for Sn, Zr,
Ti [42] and 6-311++g(d) basis set was used for C, H, O, B, and Si
atoms [36,39,43,44]. In the geometry optimization and transi-
tion-state calculations, all atoms in the simulation system were
completely relaxed and no constraints/restraints were imple-
mented. The local minima and transition states were verified by
frequency analysis and were further confirmed by intrinsic reac-
tion coordinate (IRC) calculations. To account for the effect of aque-
ous environment, calculations were performed in water dielectric
media. Enthalpies (DH) of reaction intermediates and transition
states are reported at 358 K. All the energies are reported in kcal/
mol, with respect to the ring glucose, borate salt, and Sn-beta mol-
ecules at an infinite distance.
3. Results and discussions
In an aqueous solution, at neutral pH, borate salts exist as trigo-
nal borate [B(OH)3] and as tetrahedral borate ions [B(OH)4
À
]
[45,46], in equilibrium. [B(OH)4
À
] ions have a tendency of forming
a cyclic ester complex with the cis-diol moiety in the glucose mol-
ecule [47,48]. Proton transfer from the hydroxyl groups of glucose
to the borate anion results in the formation of a rigid bidentate
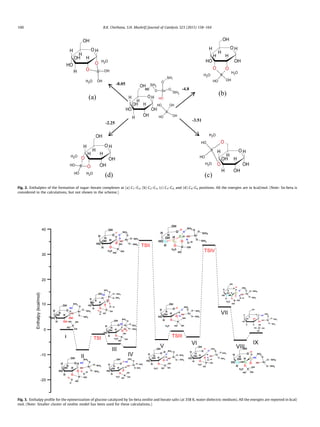
complex [49] (Fig. 2), and our calculations suggest that the forma-
tion of a sugar borate complex at C1–C2 carbon atoms of glucose is
energetically slightly more favorable than at other carbon atoms,
as shown in Fig. 2. This is consistent with the reported 13
C NMR
chemical shift values, induced on C1 (d = 103.4–104.5 ppm) to form
sugar borate complex [31]. We also computed the enthalpies of the
formation of B(OH)3 complex with glucose, and it was found to be
energetically less favorable than the glucose–borate [B(OH)4
À
] com-
plex (Details given in Fig. S1 of the supporting information). Unlike
[B(OH)4
À
], [B(OH)3] prefers to form a complex with the stannanol
group of the Sn-beta active site and with the silanol group adjacent
to Sn, through sp3
d hybridization [46,50]. We also computed the
enthalpies of formation of [B(OH)3] and [B(OH)4
À
] complexes with
Sn/Si sites of Sn-beta (Fig. S2 in the supporting information) and
observed that [B(OH)3] complexation with both Sn and Si is exo-
thermic, whereas the complexation of [B(OH)4
À
] with Sn/Si is endo-
thermic. [B(OH)3]–Sn complex is energetically the most stable
complex. The aforementioned experimental and computational
results suggest that trigonal borate [B(OH)3] prefers to form a com-
plex with Sn (Sn–O–B) and the tetrahedral borate [B(OH)4
À
] prefers
to complex with the sugar.
At neutral pH, isomerization and epimerization can occur com-
petitively; however, the epimerization reaction dominates at stoi-
chiometric or higher ratios of the borate salt [31]. 11
B NMR showed
a characteristic four-coordinate 11
B resonance peak at d = 17 ppm,
and 13
C NMR showed a resonance peak at d = 103.4, which reveal
Fig. 1. Schematic representation of the Sn-beta zeolite active site models. (a)
Smaller model cluster with little or no interaction between the Sn–OH defect site
and the silanol (Si–OH) group, and (b) larger cluster of Sn-beta zeolite model with
Sn–OH site and the neighboring silanol (Si–OH) group in close proximity. The green,
red, yellow, and white sphere represents Sn, O, Si, and H atoms, respectively. (For
interpretation of the references to color in this figure legend, the reader is referred
to the web version of this article.)
B.K. Chethana, S.H. Mushrif / Journal of Catalysis 323 (2015) 158–164 159](https://image.slidesharecdn.com/f6c6b876-32da-4622-9e91-c2803c582c43-150326220923-conversion-gate01/85/J-Catalysis-2-320.jpg)
![the existence of a sugar–borate complex in the pores of Sn-beta
zeolite [31]. Mixing glucose with borate before adding Sn-beta or
pretreating Sn-beta with borate before adding glucose did not
affect the experimental results [31]. This can be explained as fol-
lows: In the former case, sugar–borate [B(OH)4
À
] complexation
would prevent the coordination of glucose with Sn-beta, thus
inhibiting the isomerization completely, whereas, in the latter
case, trigonal borate [B(OH)3] would form a complex with Sn-beta
active sites, upon pretreating. However, after the addition of glu-
cose and the formation of sugar–borate [B(OH)4
À
] complex in the
solution, the [B(OH)3] , [B(OH)4
À
] equilibrium will shift toward
[B(OH)4
À
] ion, thus breaking the [B(OH)3]–Sn-beta complex and
making active sites of Sn-beta available for the glucose borate com-
plex. Thus, borates play a dual role in the epimerization; tetrahe-
dral [B(OH)4
À
] blocks the C1 and C2 positions of the sugar
molecule to prevent Sugar–Sn-beta complexation, and [B(OH)3]
blocks the active sites on Sn-beta (which is energetically more
favorable than Sn–O–B complex with tetrahedral borate ion), so
that pure glucose (before complexation with borate ion) cannot
access Sn-beta active sites.
To further elaborate the role of borate salts in epimerization and
to provide molecular-level details of the reaction mechanism, we
computed the Sn-beta–borate salt catalyzed epimerization of glu-
cose to mannose. The mechanism and the enthalpy profile are
shown in Fig. 3 (the corresponding free energy profile is presented
and discussed in the supporting information, Fig. S3). The epimer-
ization of glucose to mannose in the presence of catalysts Sn-beta
and borate salts involves following steps (Fig. 3): (i) formation of
the sugar–borate ester complex, (ii) glucose ring opening, (iii)
keto-enol transformation, (iv) Bilik [23] type intramolecular car-
bon–carbon rearrangement, and (v) ring closure of the epimer
product, mannose. Two different cluster models for Sn-beta, as
mentioned in Section 2, were employed.
3.1. Smaller cluster of Sn-beta zeolite model
Hydroxyl groups at C1–C2 of glucose (in the pyranose form in
water) are deprotonated by the [B(OH)4
À
] borate ion, resulting in
the formation of the sugar borate ester complex. It is accompanied
by the elimination of two water molecules, which remain coordi-
nated to borate (Fig. 3, Intermediate-II). As a result, borate forms
a bidentate complex with both O1 and O2 of glucose. Next, the
active defect site (Sn–OH) of Sn-beta abstracts a proton from the
water molecule, which is coordinated to borate, partially destabi-
lizing the sugar–borate linkage at C1 carbon, leading to the subse-
quent opening of the glucose ring (Intermediate-III). Since the ring
opening is water assisted and occurs in the presence of borate, the
activation enthalpy (6.2 kcal/mol, TSI) is lower than what is
reported before for Sn-beta [30]. This further confirms that the ring
opening is a water-assisted process [39]. Subsequently, back dona-
tion of the proton from Sn–OH2 to O5 of the acyclic glucose com-
pletes the ring opening (Intermediate-IV). In the next step, the
Si–OH (silanol) group, acting as a Brønsted acid, donates a proton
to the electrophilic keto group (C1) of the glucose to convert it into
the enol form (Intermediate-V). This is followed by an intramolec-
ular 1,2 carbon backbone rearrangement, leading to the formation
of mannose (Intermediate-VII). Finally, the hydroxyl group at C5
donates the proton to C1, converting the keto group into a hydroxyl
group, and forms the ring mannose.
Sn–OH is known to be the active site for the catalytic isomeriza-
tion reaction [30,39,51]. However, the Brønsted acidic role of Si–
OH in the Sn-beta zeolite is not well understood. Davis and co-
workers [30] suggested that the silanol hydroxyl group does not
participate in glucose isomerization, because they observed that
the catalytic activity of zeolite did not diminish even after the
exchange of hydrogen of the silanol group with Na+
ion from the
solution. In contrast, Vlachos and co-workers [36] have shown that
the direct participation of the silanol group of Sn-beta in the keto-
enol tautomerization would open up an alternate pathway for
isomerization, and thus, would reduce the activation energy bar-
rier. Our results (Fig. 3) show that Brønsted acidic Si–OH group
directly participates in the keto-enol transformation of acyclic glu-
cose, and this is a key step in the epimerization reaction. The enol
group at C1 once again forms a complex with the borate ion to
facilitate the formation of the three-member ring. However, the
activation enthalpy for the proton transfer from the silanol
group to the borate–sugar complex is found to be very high
(42.5 kcal/mol), making it appear as the rate-limiting step in the
reaction.
Like Bilik reaction, epimerization, catalyzed by Sn-beta-borate,
also proceeds through the formation of a distorted three-mem-
bered transition-state structure. This is followed by simultaneous
cleavage of the C2–C3 carbon bond and the formation of a new
C1–C3 carbon bond in the sugar. This intramolecular 1,2 carbon
shift has an activation enthalpy of 35.4 kcal/mol, 7.1 kcal/mol
lower than that for the enolization step. Contrary to our observa-
tion, the activation energy barrier for the Bilik type of rearrange-
ment, catalyzed by Sn-beta alone, was found to be slightly more
than that for the enolization by Vlachos and co-workers [36]. The
opposite trend observed in the present investigation could have
been due to the presence of the bidentate sugar–borate complex,
which facilitates the formation of the three-membered transition
state. However, another possible reason for the opposite trend
and high barriers that we observed with the implementation of a
smaller cluster was that, when Sn–O–Si bridge is cleaved in a par-
tially hydrolyzed zeolite framework, the interaction between the
stannanol and the silanol groups (which is not present in the smal-
ler cluster model) enhances the catalytic activities of both groups.
Additionally, it may also affect the binding of borate species to the
glucose molecule, thereby affecting the activation barrier for the
formation of the three-member ring.
3.2. Larger cluster of Sn-beta zeolite model
Hence, in order to investigate the effect of stannanol and silanol
interactions in a partially hydrolyzed zeolitic framework, the eno-
lization and 1,2 carbon shift steps were recalculated using a larger
cluster model for Sn-beta. With the larger cluster model, the acti-
vation enthalpy for the enolization reaction is reduced to 19.4 kcal/
mol (Fig. 4a) which is $23 kcal/mol lesser than that of the smaller
cluster model. Likewise, for Bilik mechanism also, the activation
enthalpy has been reduced to 26.3 kcal/mol (Fig. 4b) and it is
$9 kcal/mol lesser than that of the smaller cluster model. It has
to be noted that the effect of the larger cluster model and hence
of the interaction between the stannanol and the silanol group is
more pronounced on the enolization reaction. This suggests that
the interaction with the stannanol group enhances the Brønsted
acidity of the silanol group significantly and that silanol group
alone may not be able to catalyze the enolization reaction. The Bilik
type 1,2 carbon shift was found to be the rate-determining step.
The reduction in barrier for the formation of the three-member
ring also suggests that the stannanol–silanol interaction alters
the stability of intermediate VI (sugar–borate–Sn-beta complex
after enolization). These results are consistent with the experimen-
tally observed isotopic labeling studies [31]. The results suggest
that the vacancy created due to the hydrolysis of the Sn–O–Si
bridge brings about cooperative catalysis of silanol and stannanol
groups in the Sn-beta zeolite catalyst. The structural proximity of
these active groups (SnOH and SiOH) plays a pivotal role in the sta-
bilization of transition state and intermediates and hence pro-
motes the epimerization reaction.
B.K. Chethana, S.H. Mushrif / Journal of Catalysis 323 (2015) 158–164 161](https://image.slidesharecdn.com/f6c6b876-32da-4622-9e91-c2803c582c43-150326220923-conversion-gate01/85/J-Catalysis-4-320.jpg)
![3.3. Comparison of Sn/Ti/Zr activities
In order to understand the catalytic activity of the metal center
in zeolites, we replaced Sn with Ti, and Zr. The energy profile of the
rate-limiting Bilik mechanism for all three metals is shown in
Fig. 5. The computed activation enthalpies for the Bilik mechanism
are 26.3, 27.5, and 29.8 kcal/mol for Sn, Zr, and Ti, zeolite model
complexes, respectively. The results suggest that the catalytic
activity is in the following order: Sn > Zr > Ti due to the higher dia-
stereoselectivity of the Sn metal toward glucose molecules [52]. A
Fig. 4. Activation enthalpy profile for the (a) enolization and (b) Bilik mechanism using larger cluster Sn-beta zeolite model. All the energies are presented in kcal/mol.
Fig. 5. Activation enthalpy profile of the rate-limiting Bilik mechanism in the epimerization of glucose to mannose using Sn, Zr, and Ti zeolite larger cluster models. All the
energies are reported in kcal/mol.
162 B.K. Chethana, S.H. Mushrif / Journal of Catalysis 323 (2015) 158–164](https://image.slidesharecdn.com/f6c6b876-32da-4622-9e91-c2803c582c43-150326220923-conversion-gate01/85/J-Catalysis-5-320.jpg)
![similar trend was also observed when catalytic activities of these
metals were tested for the isomerization reaction [41,53]. How-
ever, it has to be noted that the Lewis acid metal center is directly
coordinated with the sugar molecule to catalyze the isomerization
reaction [53]. In the present mechanism, this is not the case. Bell
and co-workers [41] carried out an energy decomposition analysis
(EDA) to understand substrate–catalyst complex interaction for the
isomerization of glucose catalyzed by Sn-beta zeolite. They
observed that the rate-limiting hydride shift is controlled not only
by the metal active site but also by the extended zeolite environ-
ment. From EDA analysis, Bell and co-workers [41] also confirmed
that the activation energy of the reaction can be determined by
two physical properties, that is (i) Brønsted basicity of the oxygen
atom attached to the metal center, and (ii) the polarizability of the
active metal center. In case of Sn-zeolite, Sn makes the oxygen
(SnOH) atom in the stannanol group a stronger Brønsted base,
and thus, it is likely that it stabilizes the transition state (TSIV)
through electrostatic interactions. On the other hand, higher polar-
izability of Zr enables it to adjust easily to the transition-state
geometry. However, Ti lacks behind Sn and Zr in both the criteria,
and hence, exhibits the lowest activity. It has to be noted though
that the difference in activation energy barriers for these three
metals is less pronounced for epimerization since the metal center
is not directly coordinated to glucose, as in the case of
isomerization.
4. Conclusion
In summary, the epimerization reaction of glucose to mannose,
cooperatively catalyzed by the Sn-beta zeolite and borate salts, was
investigated using density functional theory calculations. In the
presence of borate salts, Sn-beta selectively performs epimeriza-
tion of glucose to mannose, since the formation of the glucose-
[B(OH)4
À
] ester complex inhibits the isomerization reaction of the
sugar. The trigonal borate [B(OH)3], through its complexation with
Sn–OH, effectively reduces the availability of free active Sn sites for
pure glucose, thereby indirectly promoting epimerization. The free
Lewis acid active site Sn–OH catalyzes the ring opening of glucose,
with explicit participation of a water molecule coordinated to the
borate ion. Additionally, the silanol group in the zeolite donates a
proton for the keto-enol transformation, thus acting as a Brønsted
acid catalyst. After the enolization, the sugar–borate complex pro-
vides a framework for the carbon backbone rearrangement and the
epimerization reaction proceeds through a mechanism similar to
that of Bilik reaction. The interaction between the silanol and the
adjacent stannanol group in the zeolite framework plays a key role
in reducing the activation barrier for the enolization step and for
the three-member ring formation, and thus, is crucial for the cata-
lytic activity of Sn-beta zeolite for sugars epimerization. Bilik
mechanism is the rate-limiting step for epimerization and requires
an activation enthalpy of 26.3 kcal/mol. Our findings also lead us to
conclude that (i) isomerization of sugars by Sn-beta zeolite can be
suppressed by the addition of borate salt and that (ii) Sn-beta zeo-
lite acts as a Lewis acid catalyst for the ring-opening reaction and
acts as a Brønsted acid catalyst for the epimerization reaction. The
catalytic activity of the different metal centers (Sn, Zr, Ti) for the
epimerization reaction is in the order Sn > Zr > Ti. The catalytic
activity depends on the Brønsted basicity of the metal oxygen
(M–OH), which stabilizes the transition state by electrostatic inter-
action and polarizability of the metal centers. We believe that
mechanistic insights in the present work would help design and
develop novel inorganic catalysts and promoters for selective epi-
merization of different sugar molecules. Similar to that of borate
salts (promoter), the molecule that has the ability to form cis-diol
complex with glucose (to inhibit isomerization) by retaining its
tetrahedral structure may be considered as a suitable promoter
for the epimerization reaction. Additionally, the sugar–promoter
complex should be small, so that it can be accommodated within
the larger pores of zeolite, since larger sugar–promoter complex
or smaller pore size of zeolite will result in a reduced yield of the
product.
Acknowledgment
Authors would like to acknowledge financial support provided
by The Ministry of Education (MOE), Singapore under the Aca-
demic Research Fund (AcRF) Tier-1 grant (Grant No. RGT36/13).
Authors would also like to thank the anonymous referee, since
his/her useful suggestions helped us improve the article.
Appendix A. Supplementary material
Complete details including free energy reaction pathway, acti-
vation energy calculations, discussion of free energy profile,
sugar–borate complex and glucose ring opening with larger cluster
model and structural data are presented in the supplementary
information. Supplementary data associated with this article can
be found, in the online version, at http://dx.doi.org/10.1016/j.jcat.
2015.01.008.
References
[1] T.K. Lindhorst, Essentials of Carbohydrate Chemistry and Biochemistry, Wiley,
2003.
[2] P.K. Ngai, T.B. Ng, Appl. Microbiol. Biotechnol. 74 (2007) 433.
[3] J.-Y. Winum, A. Scozzafava, J.-L. Montero, C.T. Supuran, Med. Res. Rev. 25
(2005) 186.
[4] D. Desai, V. Rao, H. Guo, D. Li, M. Bolgar, Int. J. Pharm. 342 (2007) 87.
[5] I. Espinosa, L. Fogelfeld, Expert Opin. Investig. Drugs 19 (2010) 285.
[6] Y. Lu, G.V. Levin, T.W. Donner, Diabetes Obes. Metab. 10 (2008) 109.
[7] K.K. Makinen, Int. J. Dent. 2010 (2010) 1.
[8] Z. Ahmed, T. Shimonishi, S.H. Bhuiyan, M. Utamura, G. Takada, K. Izumori, J.
Biosci. Bioeng. 88 (1999) 444.
[9] E.J. Moran, J.E. Tellew, Z. Zhao, R.W. Armstrong, J. Org. Chem. 58 (1993) 7848.
[10] T.E. Goodwin, K.R. Cousins, H.M. Crane, P.O. Eason, T.E. Freyaldenhoven, C.C.
Harmon, B.K. King, C.D. LaRocca, R.L. Lile, S.G. Orlicek, R.W. Pelton, O.L. Shedd,
J.S. Swanson, J.W. Thompson, J. Carbohyd. Chem. 17 (1998) 323.
[11] M. Kroger, K. Meister, R. Kava, Compr. Rev. Food Sci. Food Saf. 5 (2006) 35.
[12] M.V.S. Pinheiro, M.N. Oliveira, A.L.B. Penna, A.Y. Tamime, Int. J. Dairy Technol.
58 (2005) 193.
[13] T. Stahlberg, J.M. Woodley, A. Riisager, Catal. Sci. Technol. 2 (2012) 291.
[14] R.D. Sproull, H.C. Lim, D.R. Schneider, Biotech. Bioeng. 18 (1976) 633.
[15] M.E. Tanner, Acc. Chem. Res. 35 (2002) 237.
[16] S. Iotti, A. Sabatini, A. Vacca, J. Phys. Chem. B 114 (2010) 1985.
[17] R. Bermejo-Deval, R. Gounder, M.E. Davis, ACS Catal. 2 (2012) 2705.
[18] M.L. Hayes, N.J. Pennings, A.S. Serianni, R. Barker, J. Am. Chem. Soc. 104 (1982)
6764.
[19] V. Bilik, I. Knezek, Chem. Zvesti 44 (1990) 89.
[20] J. Feifei, V.V. David, N. Eranda, ACS Catal. 4 (2014) 1358–1364.
[21] V. Bilik, L. Petrus, V. Farkas, Chem. Zvesti 29 (1975) 690.
[22] Z. Hricoviniova, M. Hricovini, M. Petrusova, M. Matulova, L. Petrus, Chem.
Zvesti 52 (1998) 238.
[23] V. Bilik, Chem. Zvesti 26 (1972) 183.
[24] T. Tanase, F. Shimizu, M. Kuse, S. Yano, M. Hidai, S. Yoshikawa, Inorg. Chem. 27
(1988) 4085.
[25] C.-C. Chang, Z. Wang, P. Dornath, H. Je Cho, W. Fan, RSC Adv. 2 (2012) 10475.
[26] A. Corma, L.T. Nemeth, M. Renz, S. Valencia, Nature 412 (2001) 423.
[27] A. Corma, H. García, Chem. Rev. 103 (2003) 4307.
[28] A. Corma, M.E. Domine, S. Valencia, J. Catal. 215 (2003) 294.
[29] A. Corma, M. Renz, Chem. Commun. (2004) 550.
[30] R. Bermejo-Deval, R.S. Assary, E. Nikolla, M. Moliner, Y. Román-Leshkov, S.J.
Hwang, A. Palsdottir, D. Silverman, R.F. Lobo, L.A. Curtiss, M.E. Davis, Proc. Natl.
Acad. Sci. 109 (2012) 9727.
[31] W.R. Gunther, Y. Wang, Y. Ji, V.K. Michaelis, S.T. Hunt, R.G. Griffin, Y. Román-
Leshkov, Nat. Commun. 3 (2012) 1109.
[32] G. Yang, E.A. Pidko, E.J.M. Hensen, ChemSusChem 6 (2013) 1688.
[33] G. Li, E.A. Pidko, E.J.M. Hensen, Catal. Sci. Technol. 4 (2014) 2241.
[34] M. Boronat, P. Concepcion, A. Corma, M. Renz, S. Valencia, J. Catal. 234 (2005)
111.
[35] A.A. Gabrienkoa, I.G. Danilovaa, S.S. Arzumanova, A.V. Toktareva, D. Freude,
A.G. Stepanov, Micropor. Mesopor. Mater. 131 (2010) 210.
[36] N. Rai, S. Caratzoulas, D.G. Vlachos, ACS Catal. 3 (2013) 2294.
[37] A.D. Becke, J. Chem. Phys. 98 (1993) 1372.
B.K. Chethana, S.H. Mushrif / Journal of Catalysis 323 (2015) 158–164 163](https://image.slidesharecdn.com/f6c6b876-32da-4622-9e91-c2803c582c43-150326220923-conversion-gate01/85/J-Catalysis-6-320.jpg)
![[38] A.D. Becke, J. Chem. Phys. 98 (1993) 5648.
[39] R.S. Assary, L.A. Curtiss, Energy Fuels 26 (2012) 1344.
[40] M.J. Frisch, G.W. Trucks, H.B. Schlegel, M.A.R.G.E. Scuseria, J.R. Cheeseman, G.
Scalmani, V. Barone, B. Mennucci, G.A. Petersson, H. Nakatsuji, M. Caricato, X.
Li, H.P. Hratchian, A.F. Izmaylov, J. Bloino, G. Zheng, J.L. Sonnenberg, M. Hada,
M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda,
O. Kitao, H. Nakai, T. Vreven, J.J.A. Montgomery, J.E. Peralta, F. Ogliaro, M.
Bearpark, J.J. Heyd, E. Brothers, K.N. Kudin, V.N. Staroverov, R. Kobayashi, J.
Normand, K. Raghavachari, A. Rendell, J.C. Burant, S.S. Iyengar, J. Tomasi, M.
Cossi, N. Rega, J.M. Millam, M. Klene, J.E. Knox, J.B. Cross, V. Bakken, C. Adamo,
J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C.
Pomelli, J.W. Ochterski, R.L. Martin, K. Morokuma, V.G. Zakrzewski, G.A. Voth,
P. Salvador, J.J. Dannenberg, S. Dapprich, A.D. Daniels, Ö. Farkas, J.B. Foresman,
J.V. Ortiz, J. Cioslowski, D.J. Fox, Gaussian Inc., Wallingford, CT, 2009.
[41] Y.-P. Li, M. Head-Gordon, A.T. Bell, ACS Catal. 4 (2014) 1537.
[42] W.R. Wadt, P.J. Hay, J. Chem. Phys. 82 (1985) 284.
[43] T. Ståhlberg, S. Rodriguez-Rodriguez, P. Fristrup, A. Riisager, Chem. Eur. J. 17
(2011) 1456.
[44] V. Choudhary, S. Caratzoulas, D.G. Vlachos, Carbohyd. Res. 368 (2013) 89.
[45] J. Quirino, J. Chromatogr. 72 (2010) 503.
[46] W.R. Gunter, Q. Duong, Y. Roman-Leshkov, J. Mol. Catal. A: Chem. 379 (2013)
294.
[47] S. Chapelle, J.-F. Verchere, Tetrahedron 44 (1988) 4469.
[48] J.F. Verchere, M. Hlaibi, Polyhedron 6 (1987) 1415.
[49] R. Van den Berg, J.A. Peters, H. Van Bekkum, Carbohyd. Res. 253 (1994) 1.
[50] A.D. Irwin, J.S. Holmgren, T.W. Zerda, J. Jonas, J. Non-Cryst. Solids 89 (1987)
191.
[51] J. Dijkmans, D. Gabriels, M. Dusselier, F. de Clippel, P. Vanelderen, K.
Houthoofd, A. Malfliet, Y. Pontikes, B.F. Sels, Green Chem. 15 (2013) 2777.
[52] S. Shetty, S. Pal, D.G. Kanhere, A. Goursot, Chem. Eur. J. 12 (2005) 518.
[53] R.S. Assary, L.A. Curtiss, J. Phys. Chem. A 115 (2011) 8754.
164 B.K. Chethana, S.H. Mushrif / Journal of Catalysis 323 (2015) 158–164](https://image.slidesharecdn.com/f6c6b876-32da-4622-9e91-c2803c582c43-150326220923-conversion-gate01/85/J-Catalysis-7-320.jpg)