This document provides a nutrition assessment and medical nutrition therapy plan for a 63-year-old male patient who underwent a laryngectomy, pharyngectomy, esophagectomy with gastric pull-up followed by radiation for squamous cell carcinoma. The registered dietitian found the patient to be at high nutritional risk due to a 23 pound unintended weight loss over the past year and being 79% of his ideal body weight at the time of surgery. The initial plan was to start enteral nutrition via tube feeding post-operatively. Follow-up notes document the patient tolerating the tube feeding well with some loose stools. The plan was adjusted to a fiber-containing formula and supplements to address this. The registered diet
![Megan McDermett
March 28, 2016
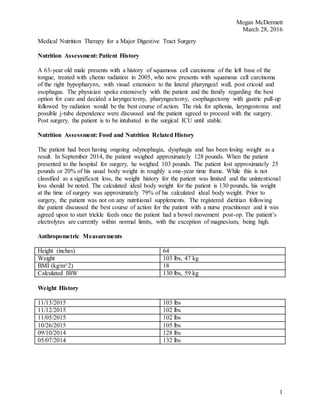
2
Labs
Albumin 3.0 L 11/05/2015
Anion Gap 4.0 11/13/2015
BUN 24 11/13/2015
Ca 8.1 11/13/2015
Cl 102 11/13/2015
CO2 30 11/13/2015
Creatinine 1.0 11/13/2015
Glucose 128 H 11/13/2015
K 3.9 11/13/2015
Na 136 11/13/2015
PO4 4.4 11/13/2015
eGFR 80.2 11/13/2015
SGOT 19 11/05/2015
SGPT 23 11/05/2015
Hgb A1c 4.9 05/07/2014
Mg 3.4 H 11/13/2015
Alk Phos 88 11/05/2015
Estimated Energy Requirements
Energy: 35-40 kcal/kg actual BW = 1700-1900 kcal/day
Protein: 1.5-2.0 gm/kg actual BW = 70-95 gm/day
Fluids: 1 ml/kcal = >1700 mls/day
Nutrition Diagnoses
1) Unintentional weight loss (NC-3.2 [Clinical category from the Academy of Nutrition and
Dietetics’ Nutrition Care Process terminology]) as related to odynophagia and dysphagia
secondary to squamous cell carcinoma of the hypopharynx, cervical esophagus as
evidenced by a noted 23 pound weight loss over the last year.
2) Increased nutrient (protein and kcal) needs (NI-5.1 [Nutrient Intake category from the
Academy of Nutrition and Dietetics’ Nutrition Care Process terminology]) related to need
for healing post-op as evidenced by status post laryngophareygectomy, transhiatal
esophagectomy/j-tube placement with gastric pull-up.
Nutrition Intervention: Prescription/Plan
Initiate trickle feeds of Pivot 1.5 and advance to goal rate of 45 ml/hour continuous as patient
tolerates tube feeding. The goal rate of tube feeding provides 1620 kcals, 101 gm pro (2.1
gm/kg), and 820 mls free water. Additional free water autoflushes at 25 mls/hour will give the
patient 1420 mls of free water.](https://image.slidesharecdn.com/23948b47-a3e5-4789-9455-909ab75c55cd-161212203137/85/ISPEN-MNT-Case-Study-2-320.jpg)
![Megan McDermett
March 28, 2016
3
Nutrition Intervention: MNT Goals
1) For the patient to tolerate the tube feeding so as to promote positive nutritional status
post-op and surgical wound healing.
2) For the patients electrolytes to be within normal limits status-post tube feeding initiation
so as to prevent refeeding syndrome.
3) For the patient to have normal bowel function post op so as to avoid post op ileus and
further decline in nutritional status.
Nutrition Monitoring/Evaluation
Dietitian will monitor patient’s weight, labs, electrolyte levels (magnesium, potassium, and
phosphorus), tube feeding tolerance, and overall hospital course. Patient’s nutritional status noted
as “severely compromised” and the patient will be reassessed within 3-5 days per hospital policy
unless a nutrition follow up is indicated anytime prior to 3-5 days.
Nutrition Status
This patient has been marked as “severely compromised” based on entire nutrition assessment.
Follow up Nutrition Assessment
Patient is a 63-year-old male status-post laryngopharyngectomy, transhiatal esophagectomy/j-
tube placement with gastric pull-up for laryngeal squamous cell carcinoma with pharyngeal and
cervical esophageal extension. Patient communicating via “Boogie Board” eWriter. Patient
reports to be tolerating tube feeding well and denies any gastrointestinal discomfort. He reports
some loose stool, but otherwise all previous goals are being met. Noted that patient is also
receiving docusate, omeprazole, ondanseteron, and calcitriol.
Follow up Estimated Energy Requirements
Energy: 35-40 kcal/kg actual BW = 1700-1900 kcal/day
Protein: 1.5-2.0 gm/kg actual BW = 70-95 gm/day
Fluids: 1 ml/kcal = >1700 mls/day
(no change since initial assessment)
Follow up Nutrition Diagnoses
1) Unintentional weight loss (NC-3.2 [Clinical category from the Academy of Nutrition and
Dietetics’ Nutrition Care Process terminology]) as related to odynophagia and dysphagia
secondary to squamous cell carcinoma of the hypopharynx, cervical esophagus as
evidenced by noted 23 pound weight loss over the last year.
2) Increased nutrient (protein and kcal) needs (NI-5.1 [Nutrient Intake category from the
Academy of Nutrition and Dietetics’ Nutrition Care Process terminology]) related to need
for healing post-op as evidenced by status post laryngophareygectomy, transhiatal](https://image.slidesharecdn.com/23948b47-a3e5-4789-9455-909ab75c55cd-161212203137/85/ISPEN-MNT-Case-Study-3-320.jpg)






