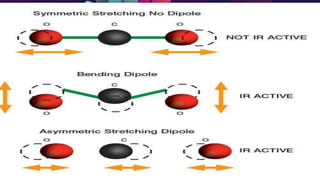
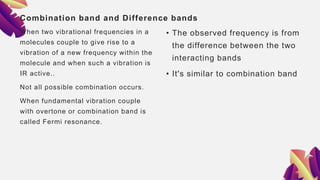
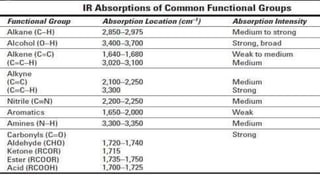
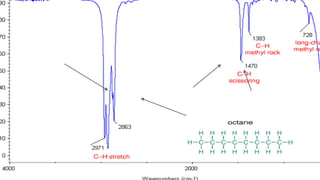
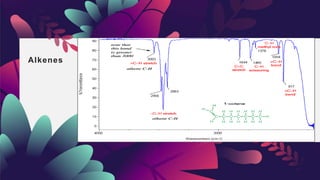
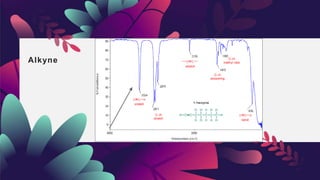
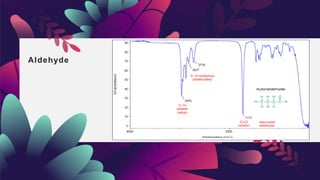
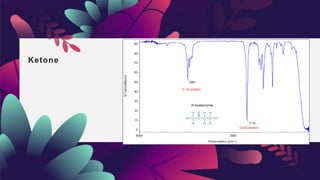
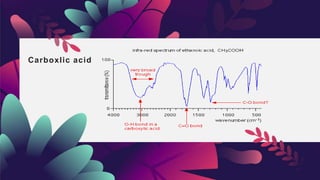
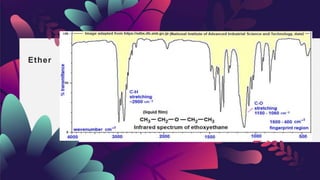
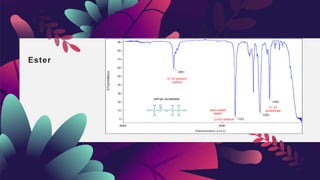
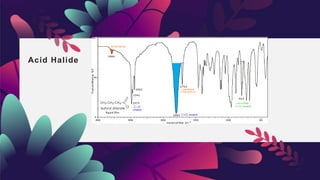
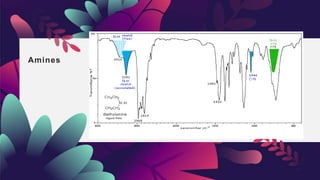
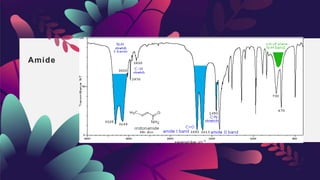
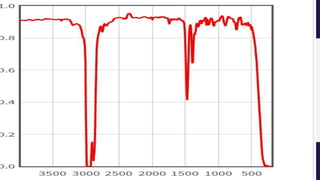
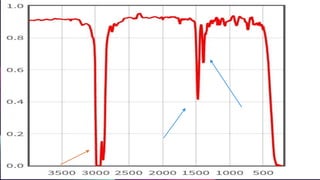
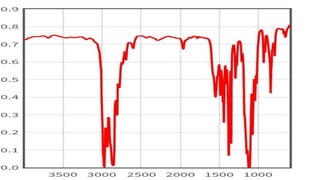
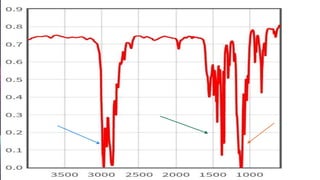
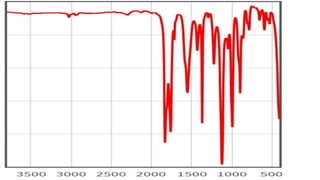
This document provides an overview of infrared spectroscopy. It discusses how IR spectroscopy can be used to determine functional groups present in molecules by analyzing absorption of infrared light. Different types of vibrational motions that molecules undergo are described, including bending and stretching vibrations. Factors that influence IR spectra, such as hybridization and resonance, are also covered. Preparation of samples for IR analysis and basic components of IR spectrometers are outlined. Finally, some example IR spectra are presented to demonstrate identification of functional groups.