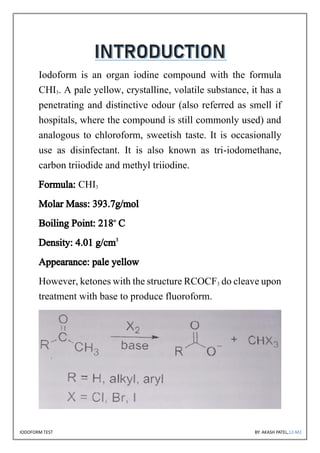
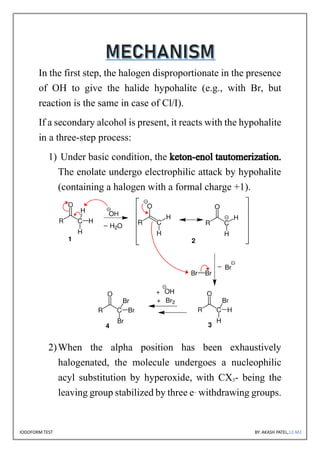
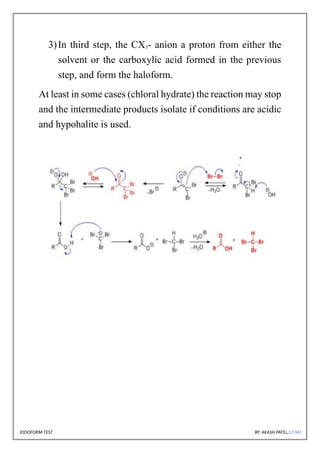
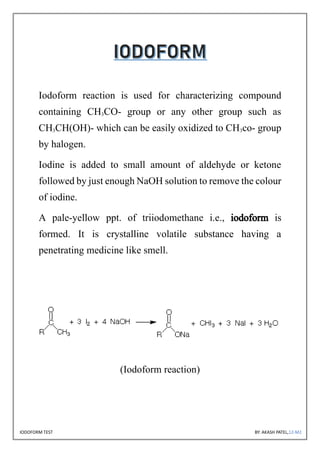
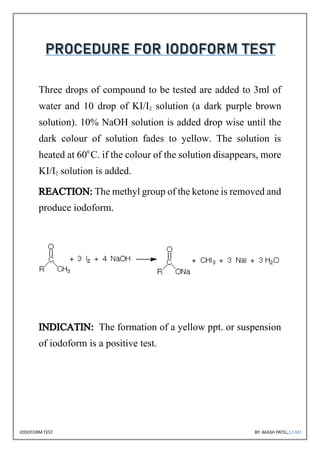
The document discusses the iodoform test, a chemical procedure used to identify the presence of methyl ketones through a reaction that produces a yellow precipitate of iodoform. It includes detailed information on the chemical properties of iodoform, the reaction procedure, its laboratory, industrial, and medical uses, as well as potential side effects. The project is authored by Akash Patel, certified by his school, and acknowledges the guidance received during the project.