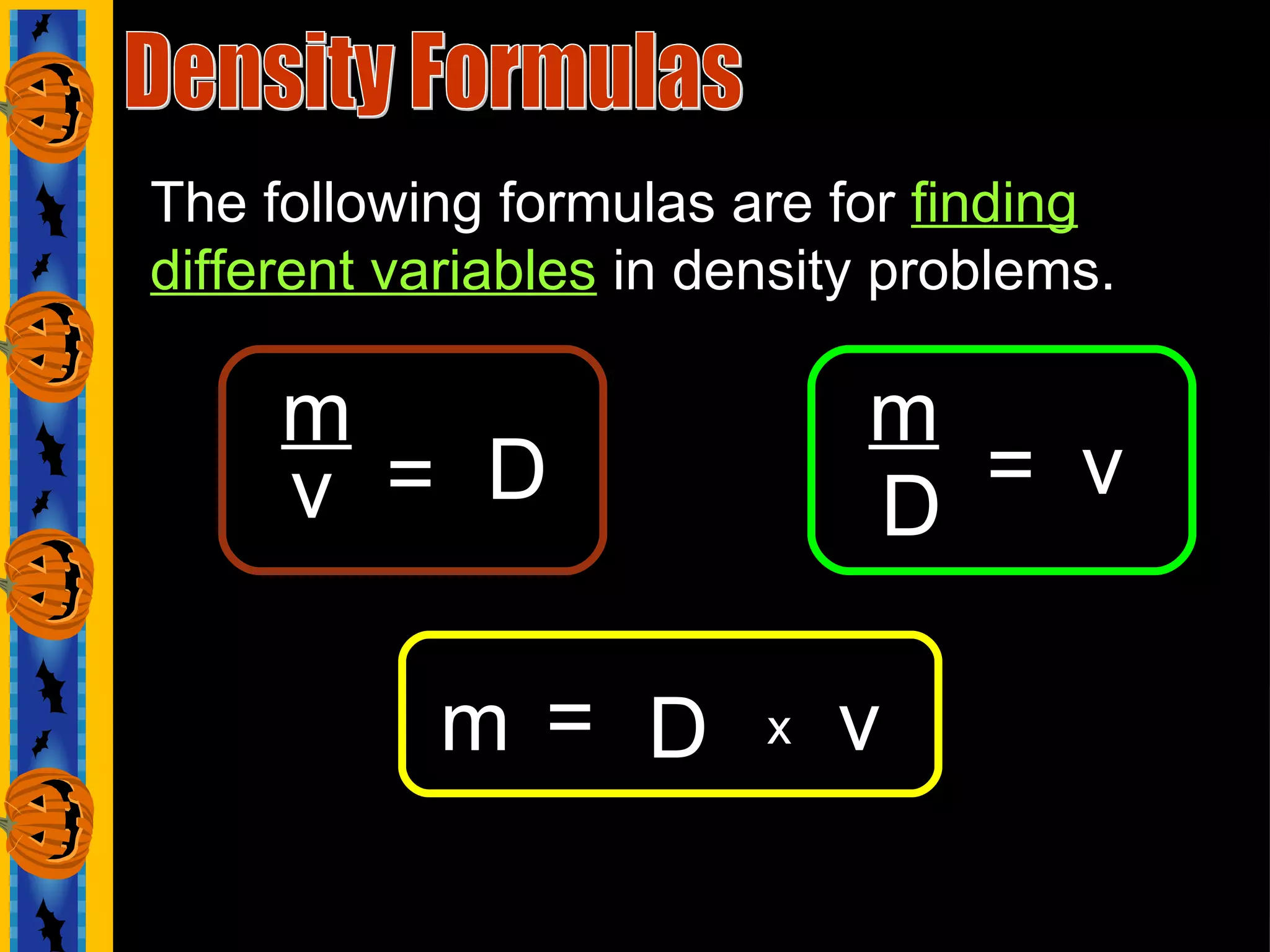
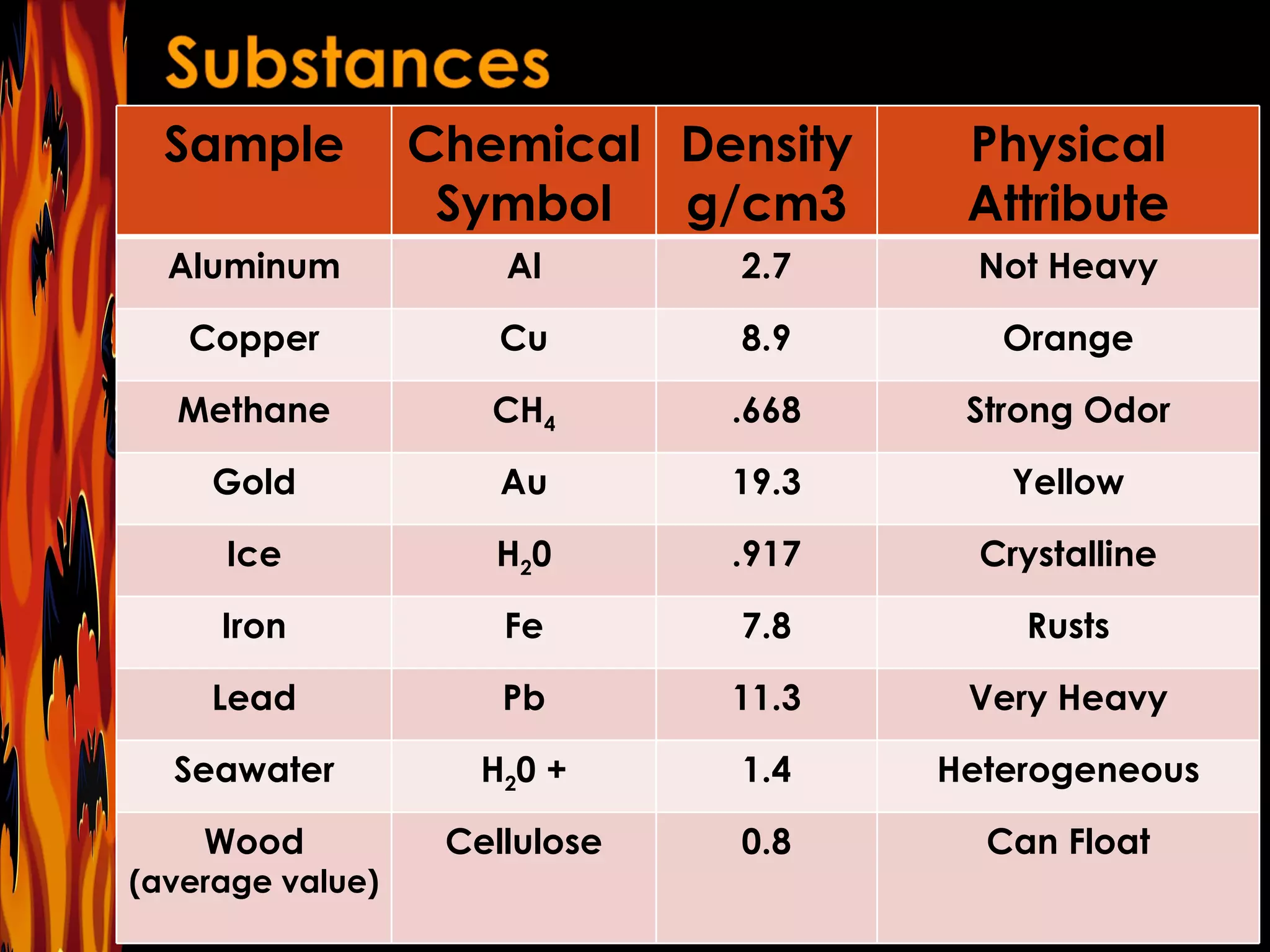
The document contains questions related to density, mass, volume, and formulas used to calculate these values. It asks about the units used to measure and identify different physical properties. It provides examples of chemical symbols, densities in g/cm3, and physical attributes of various substances like aluminum, copper, gold, ice, and seawater. It includes formulas for calculating density, mass, and volume and asks students to identify which formula to use given values.