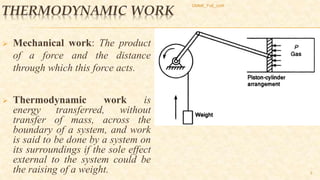
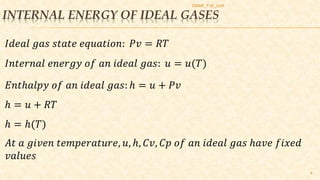
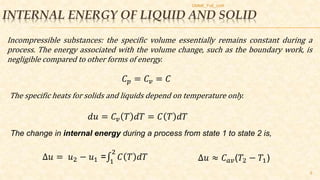
This document discusses thermodynamic concepts including internal energy, enthalpy, and thermodynamic work. It provides definitions and equations for calculating the internal energy and enthalpy of ideal gases, liquids, and solids. For ideal gases, the internal energy and enthalpy depend only on temperature. For incompressible liquids and solids, the specific heat is constant and the change in internal energy can be calculated using the average temperature. The document includes two example problems applying these concepts to calculate temperature changes in systems.