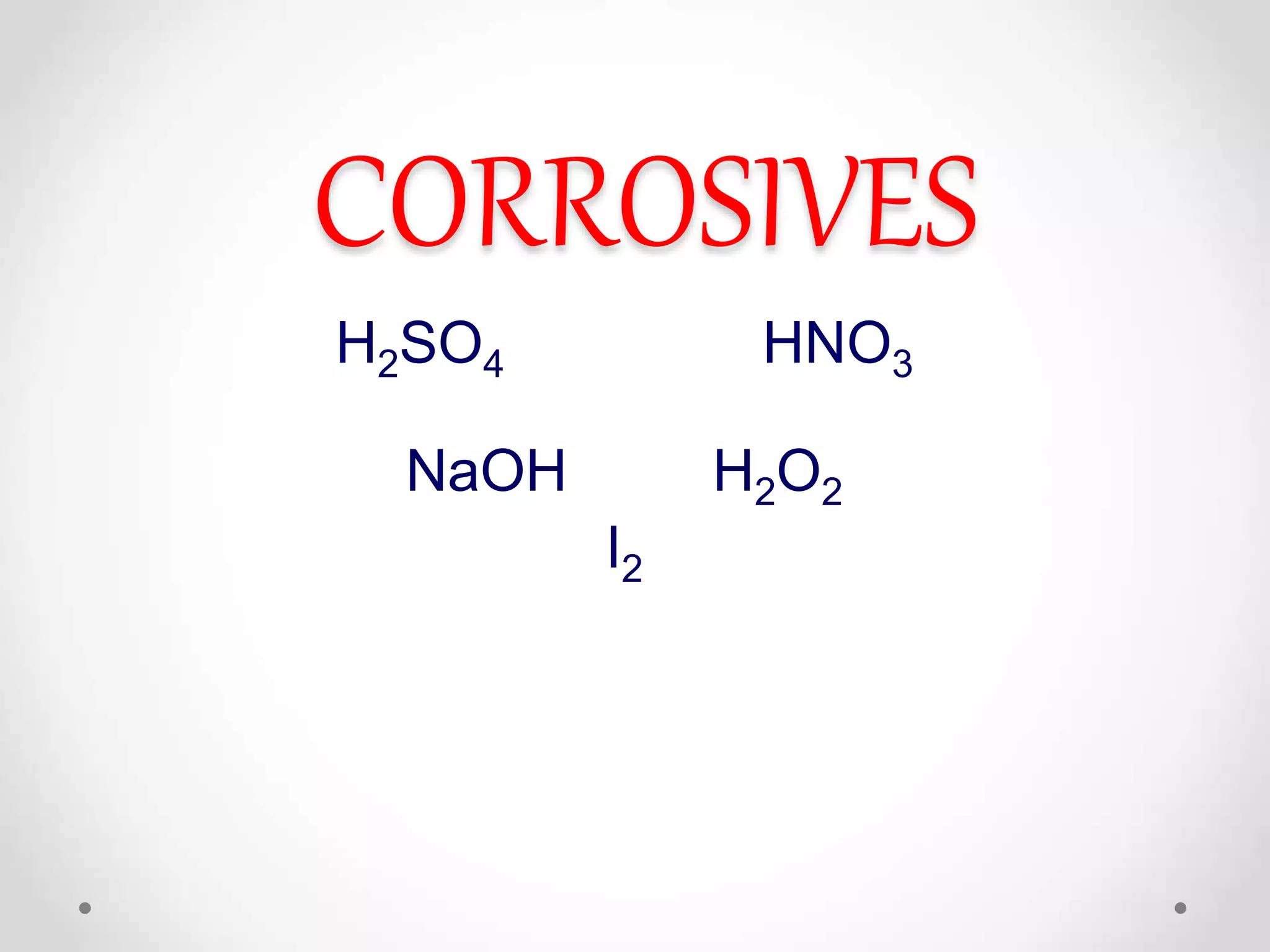
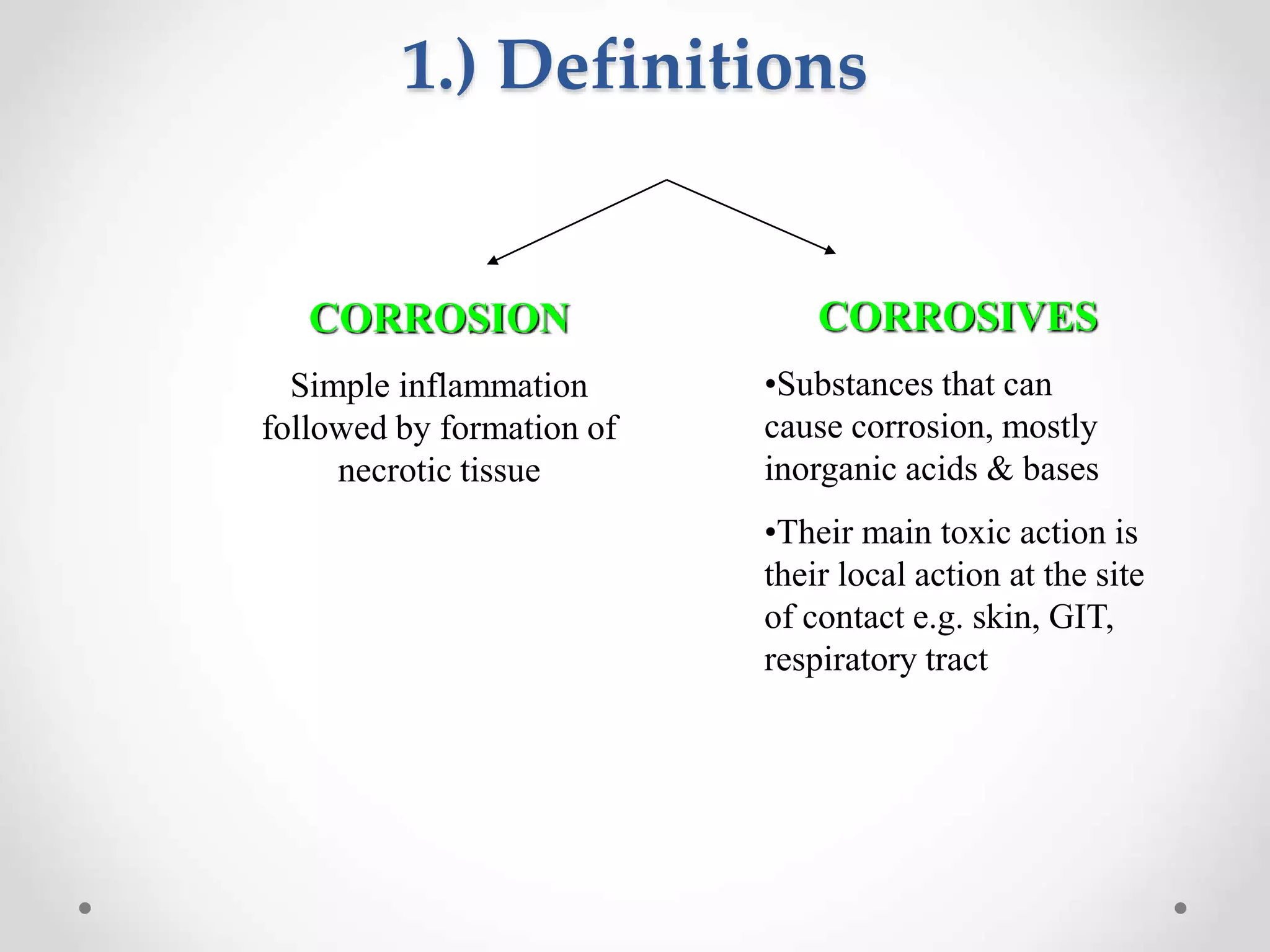
This document summarizes different classes of corrosive substances including their physical properties, mechanisms of corrosion, clinical manifestations, management, and effects on the intestine. It describes acids like sulfuric and nitric acid, bases like sodium hydroxide, and oxidants like hydrogen peroxide and iodine. Each corrosive is defined with its characteristics like viscosity, odor, and color. Their mechanisms involve reactions with proteins, hemoglobin, lipids that can cause liquefaction, discoloration, or saponification of tissues. Clinical effects vary by site of contact and can include burning, vomiting, perforation, edema, and destruction of the esophagus, stomach, eyes or skin. Management involves dilution, demulcents