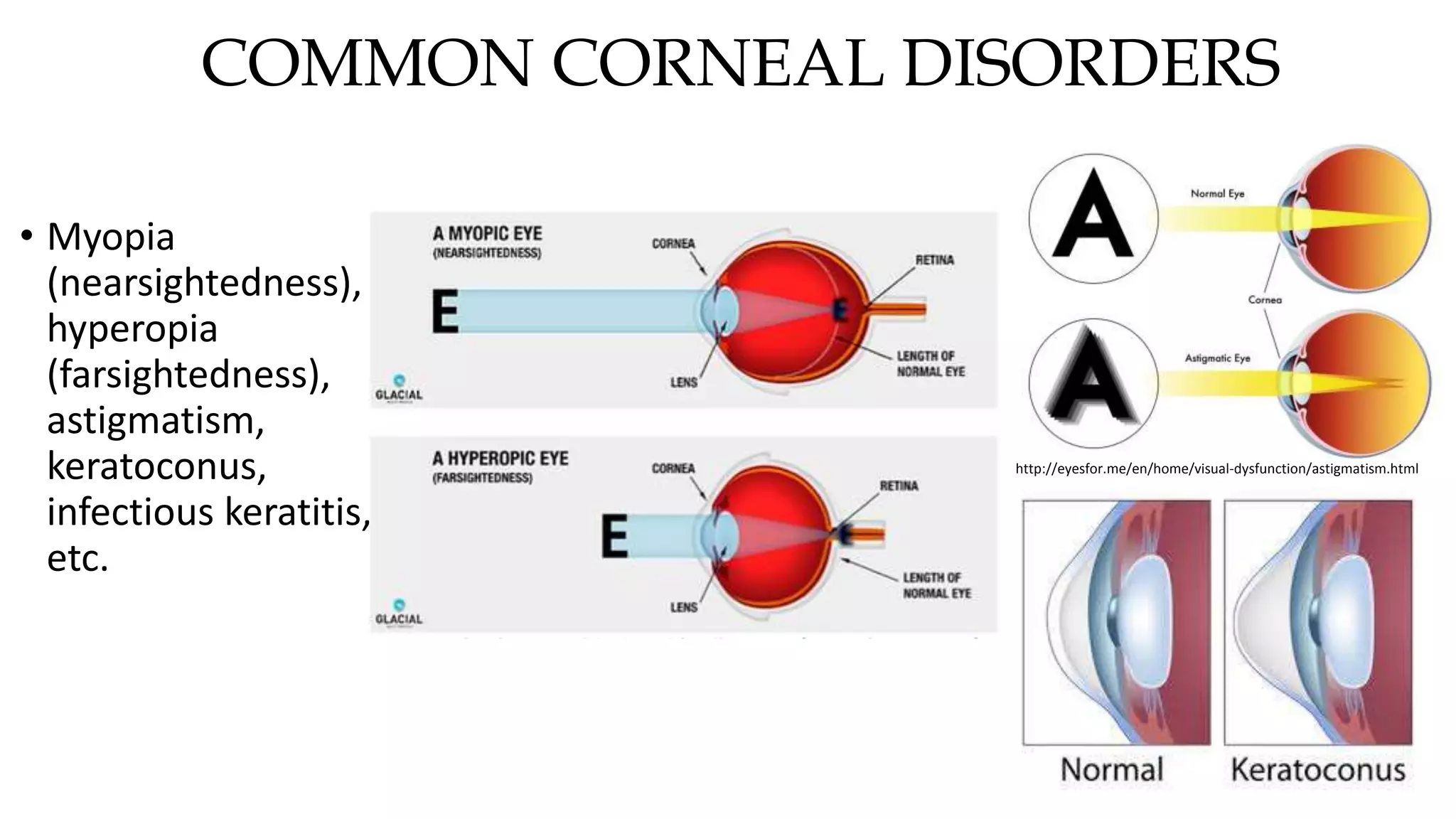
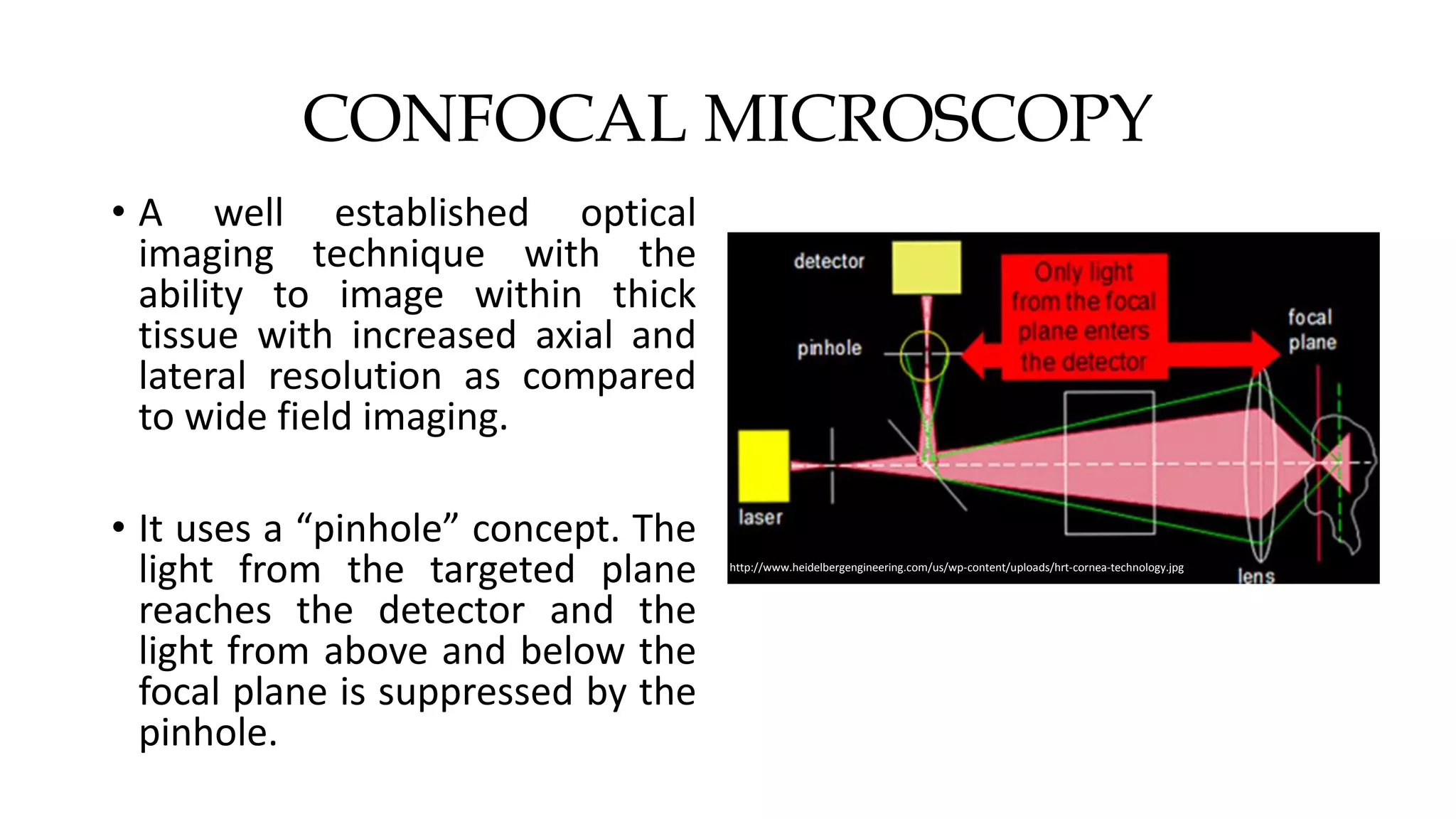
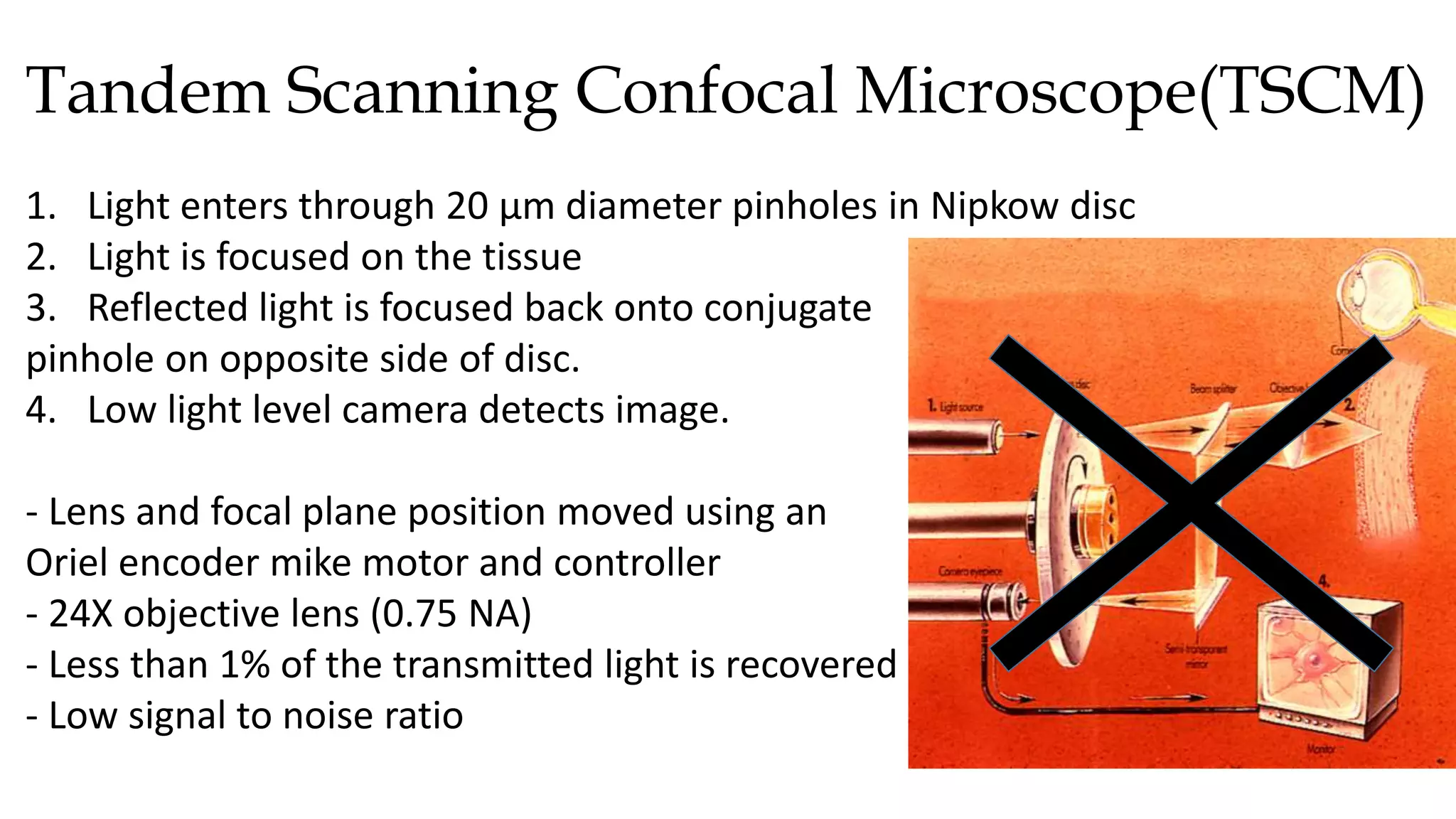
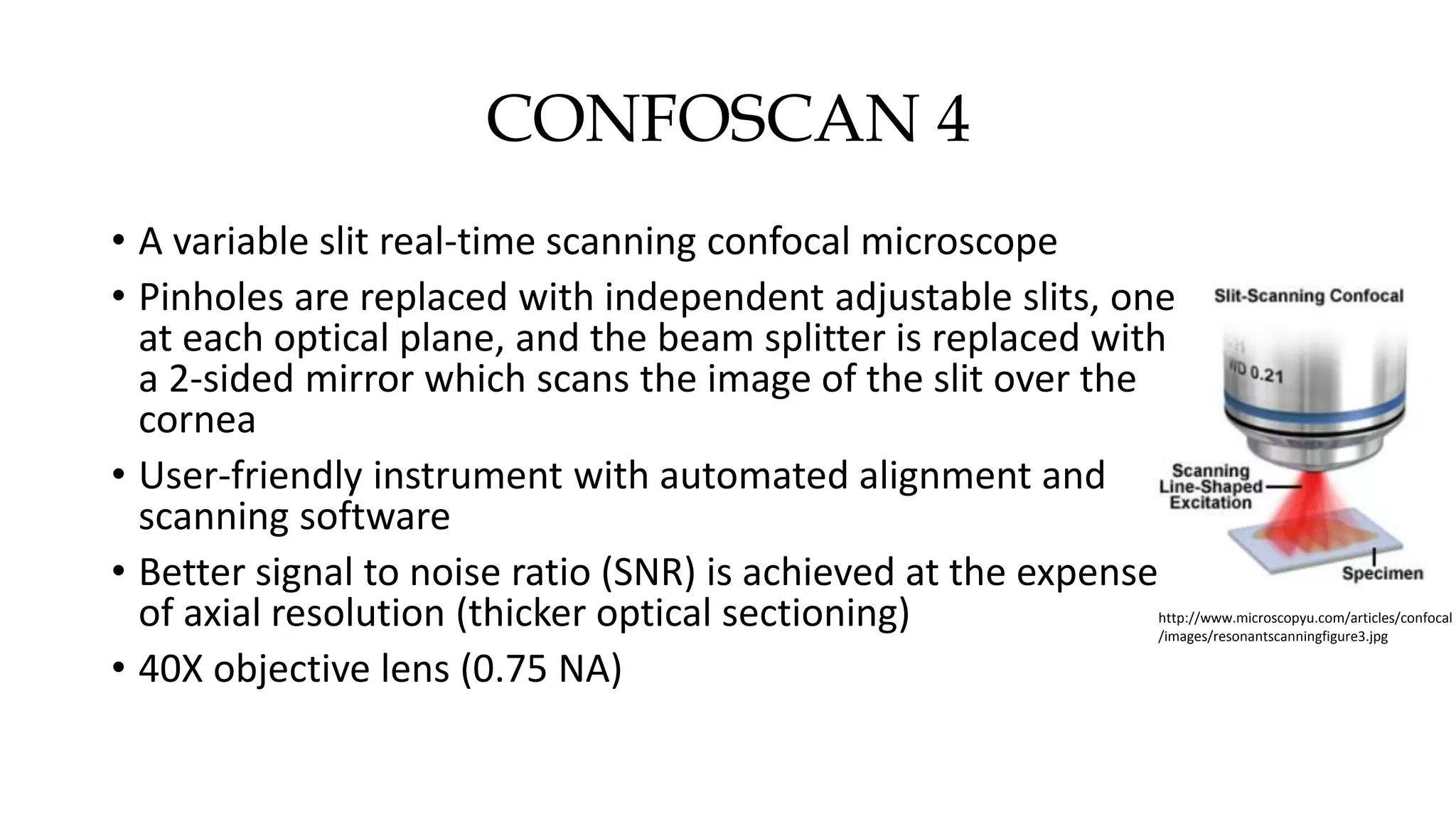
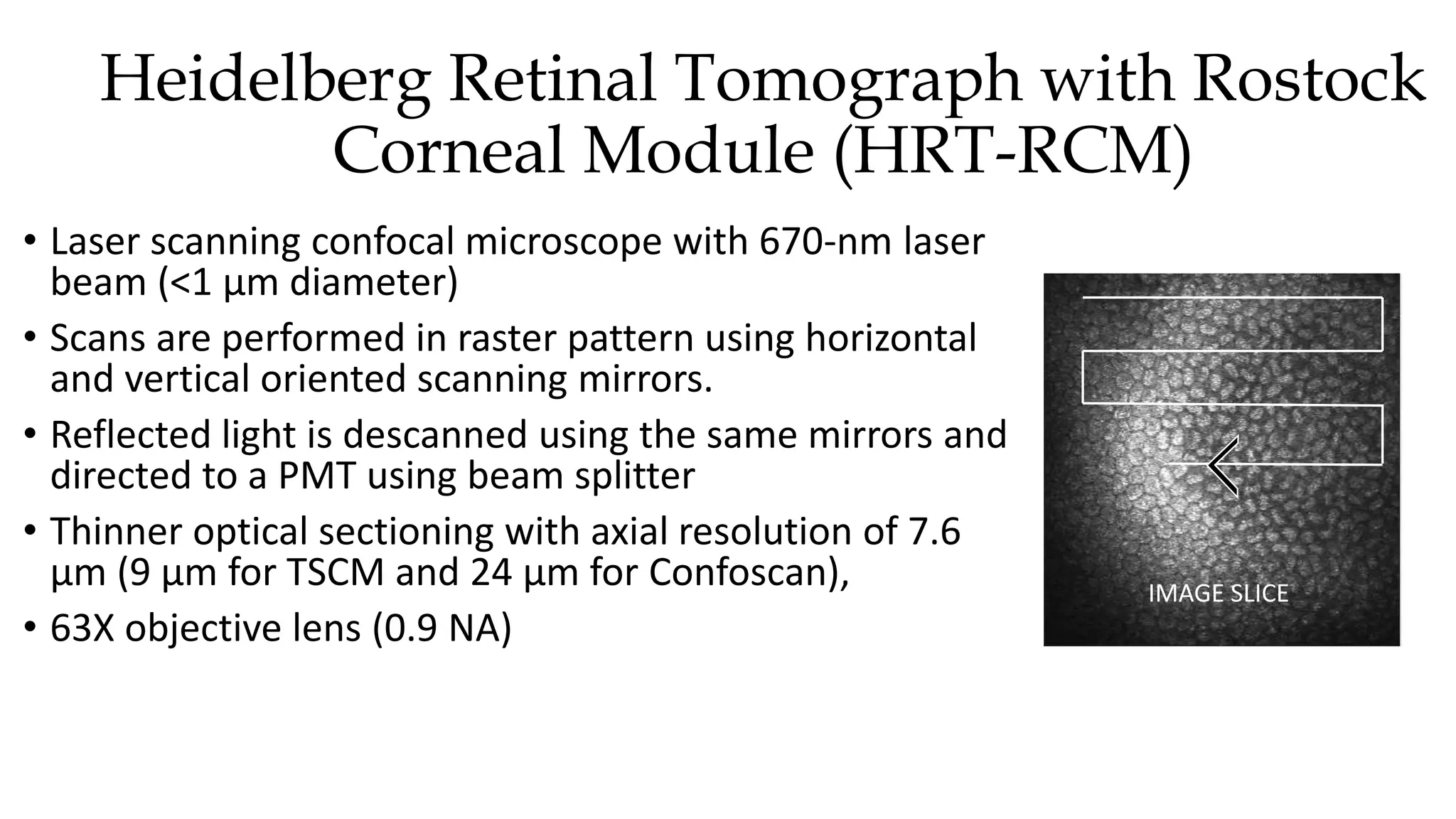
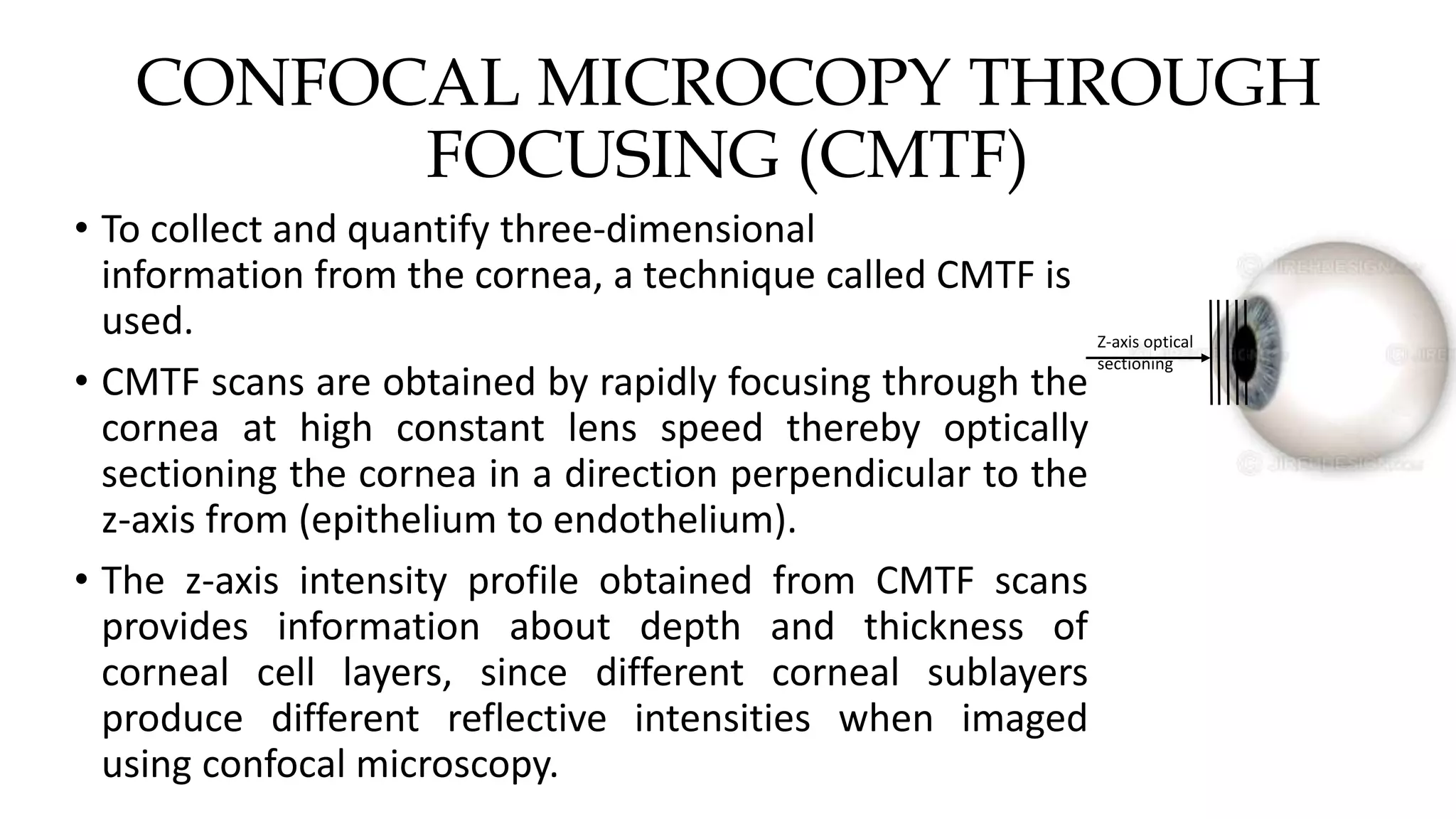
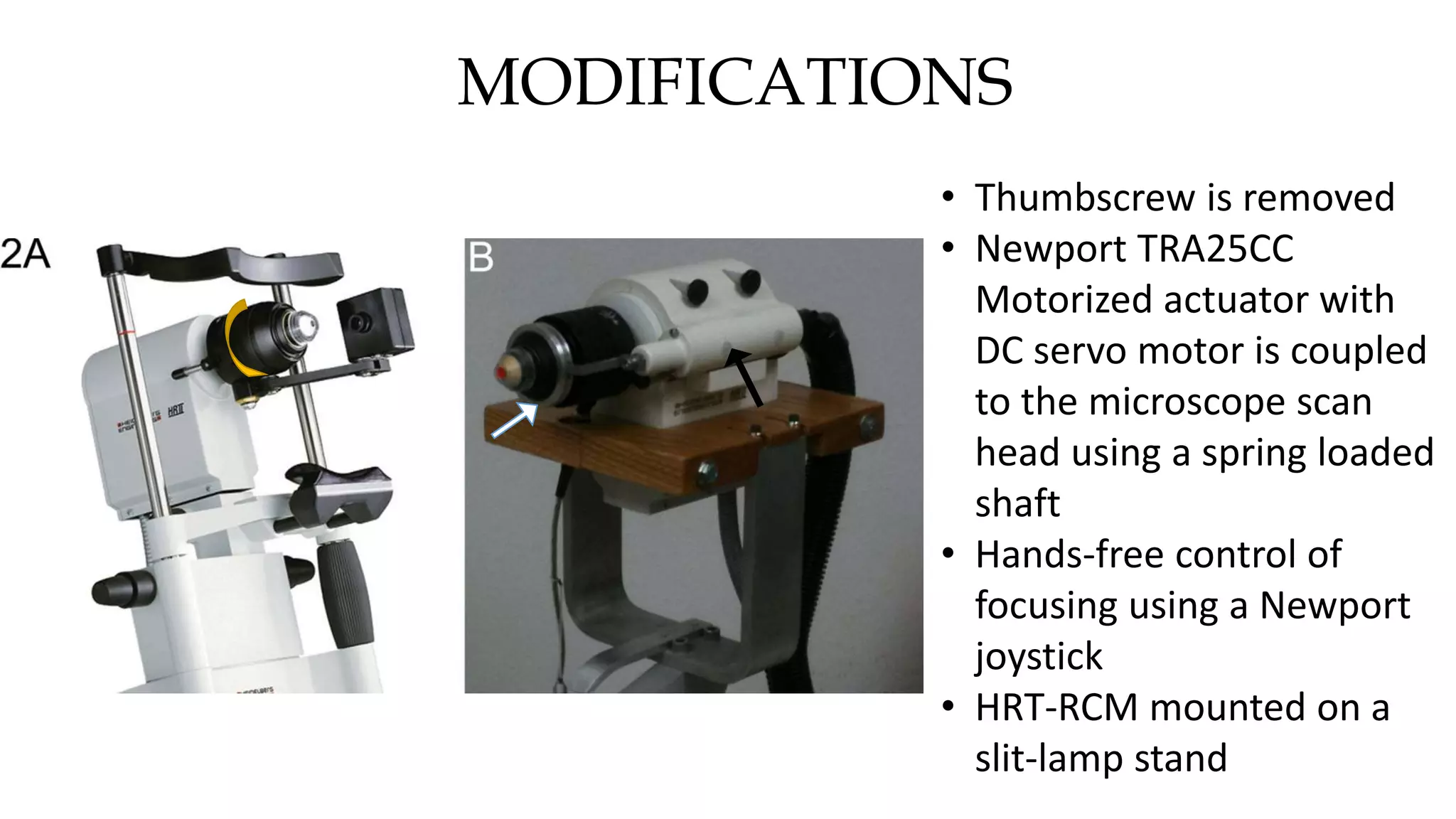
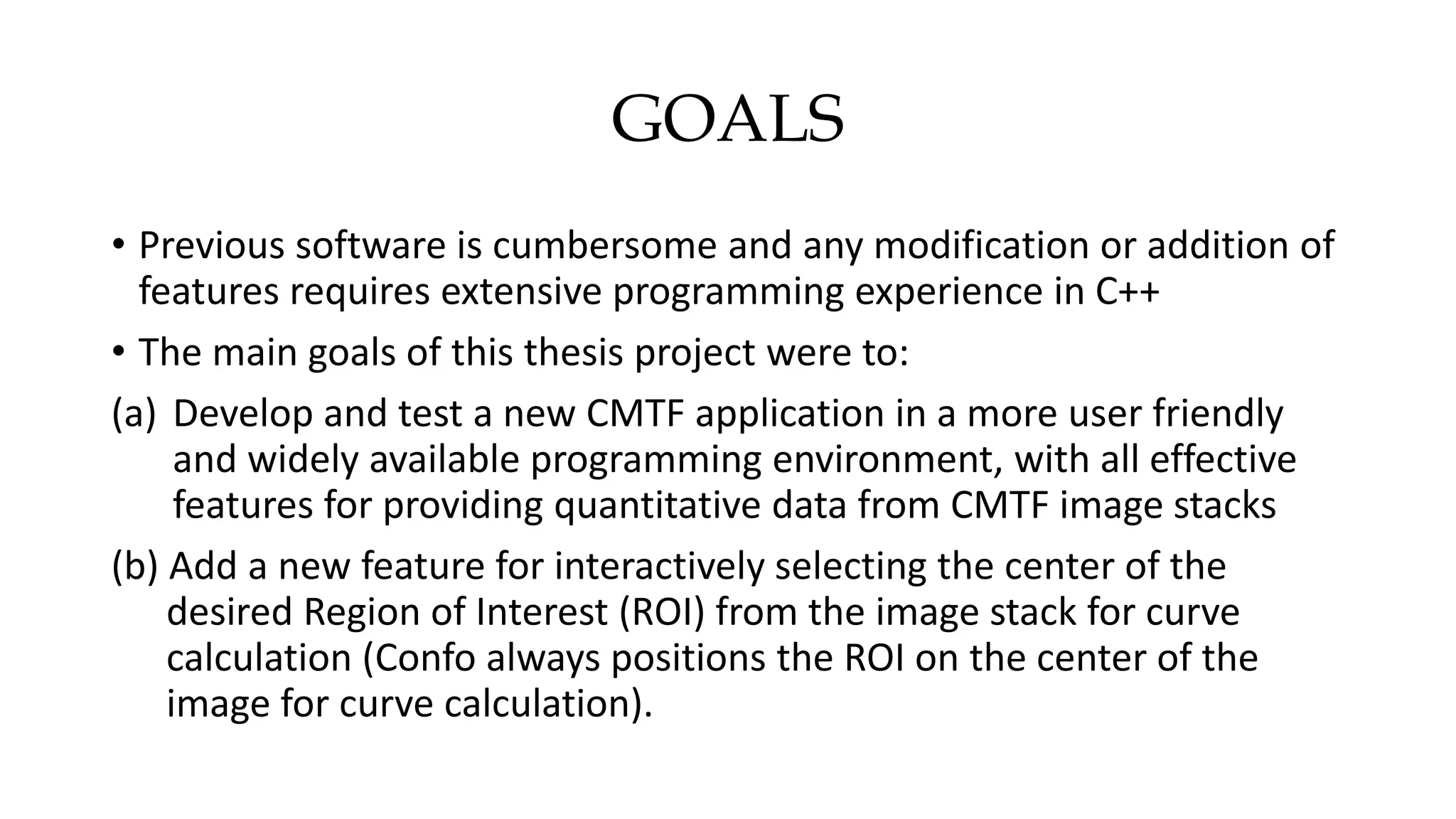
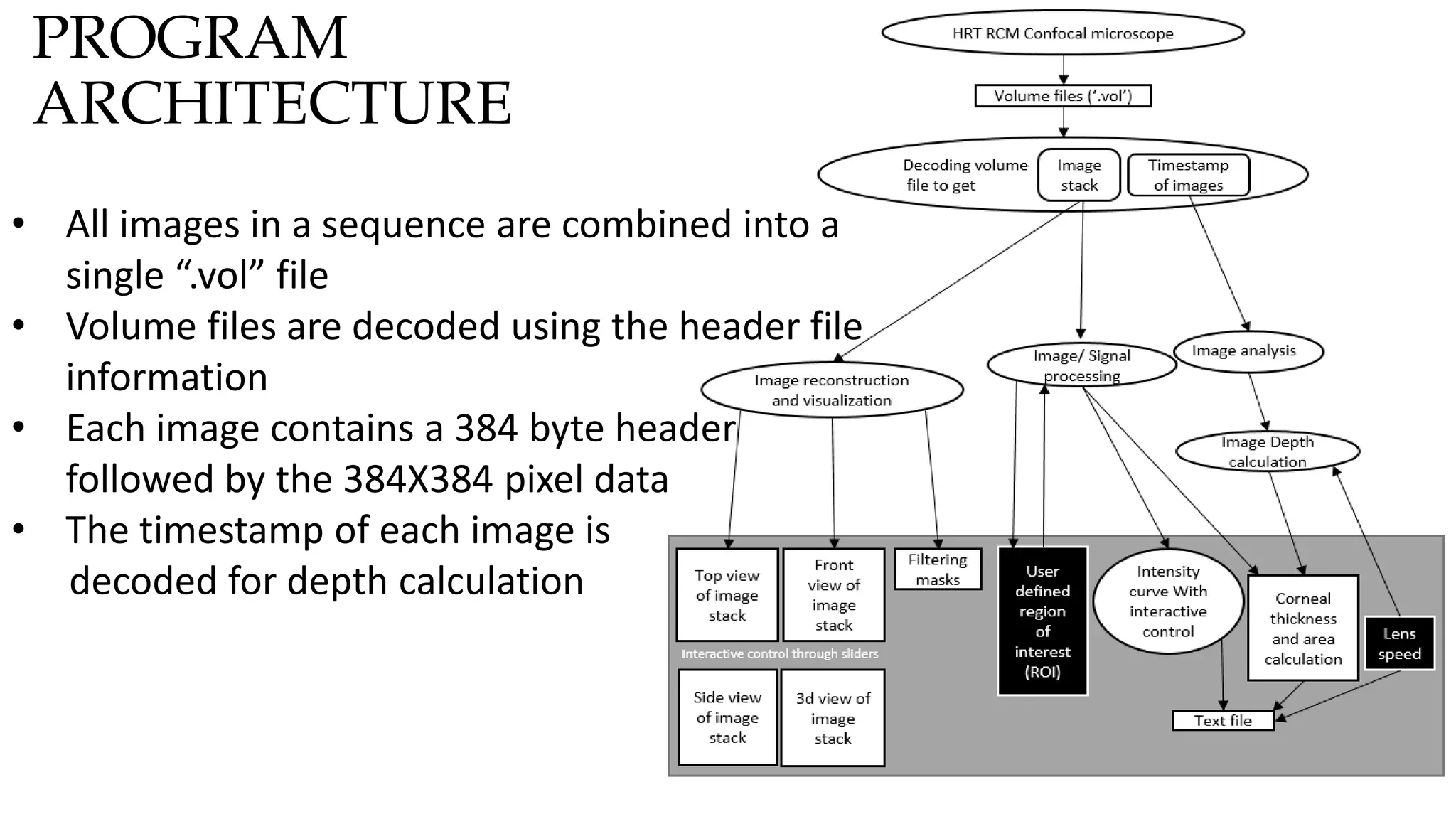
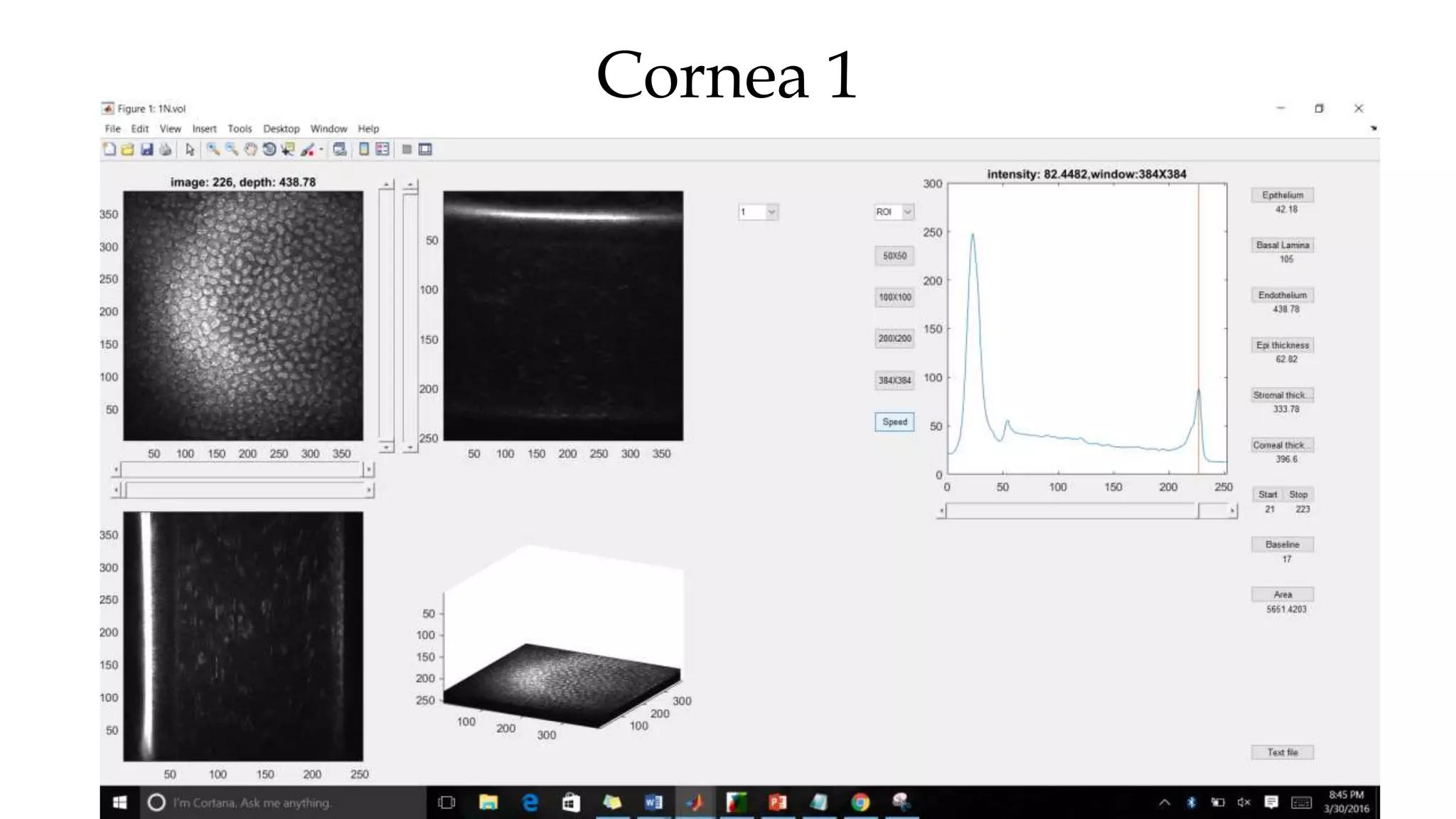
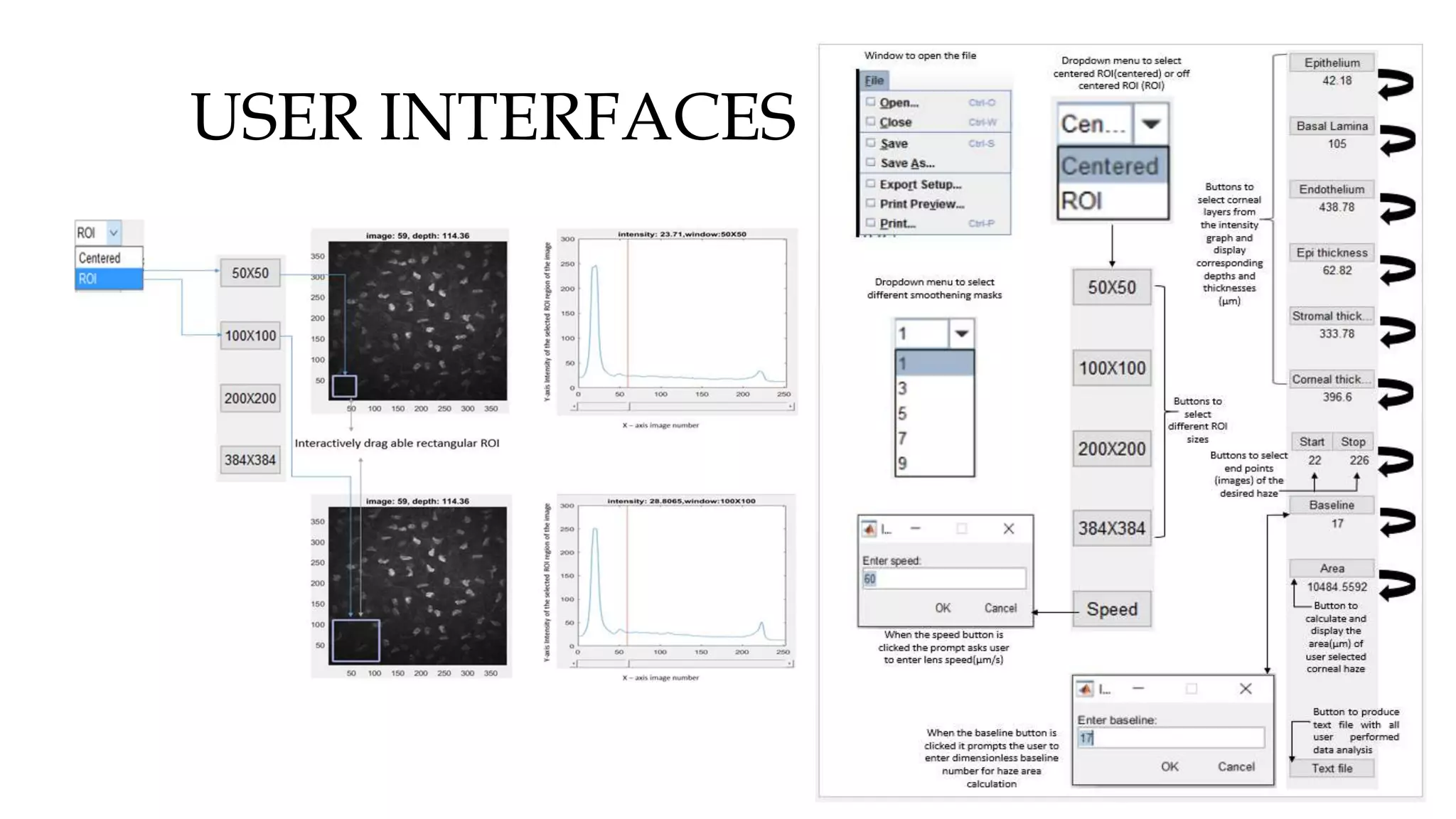
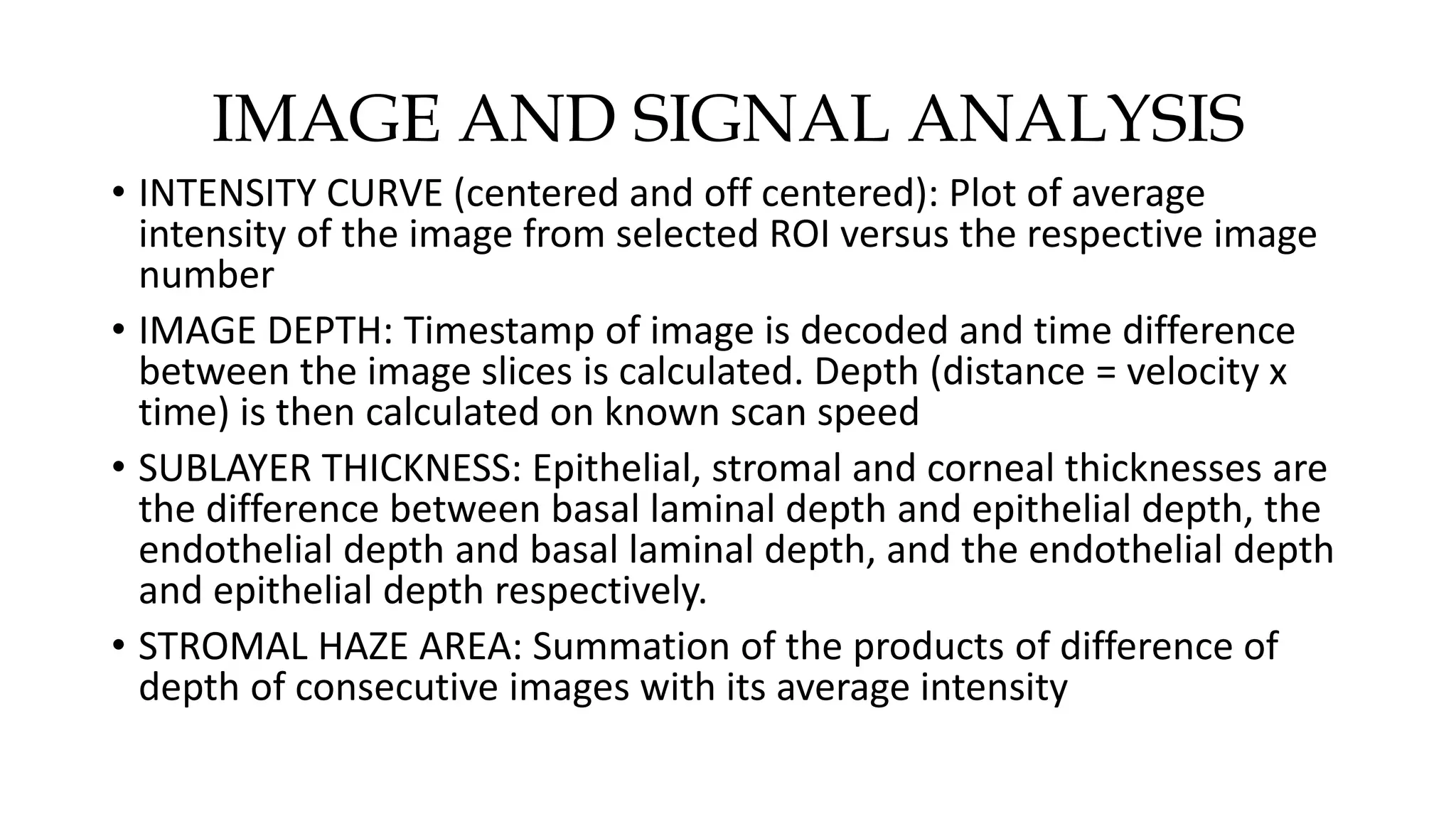
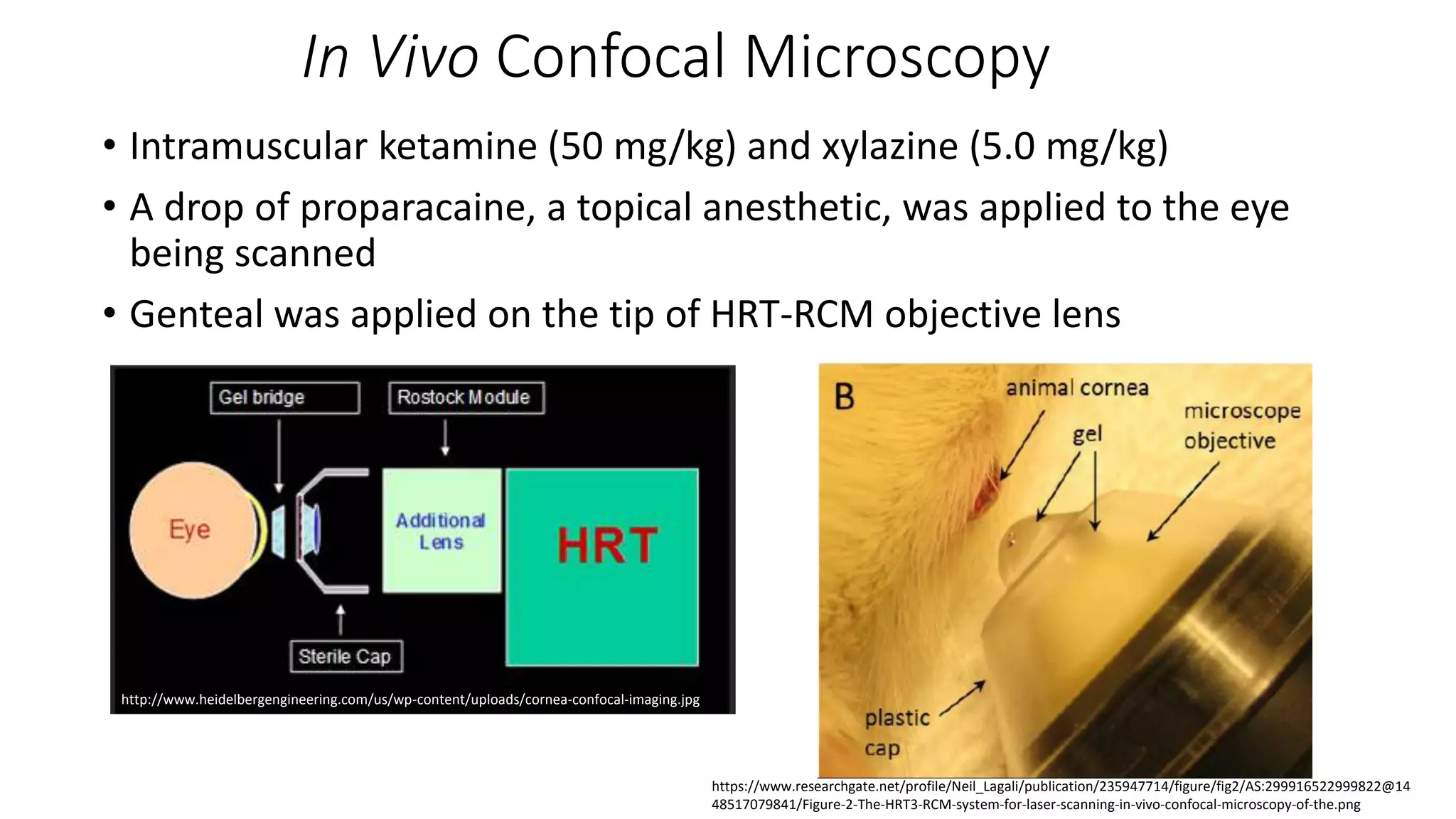
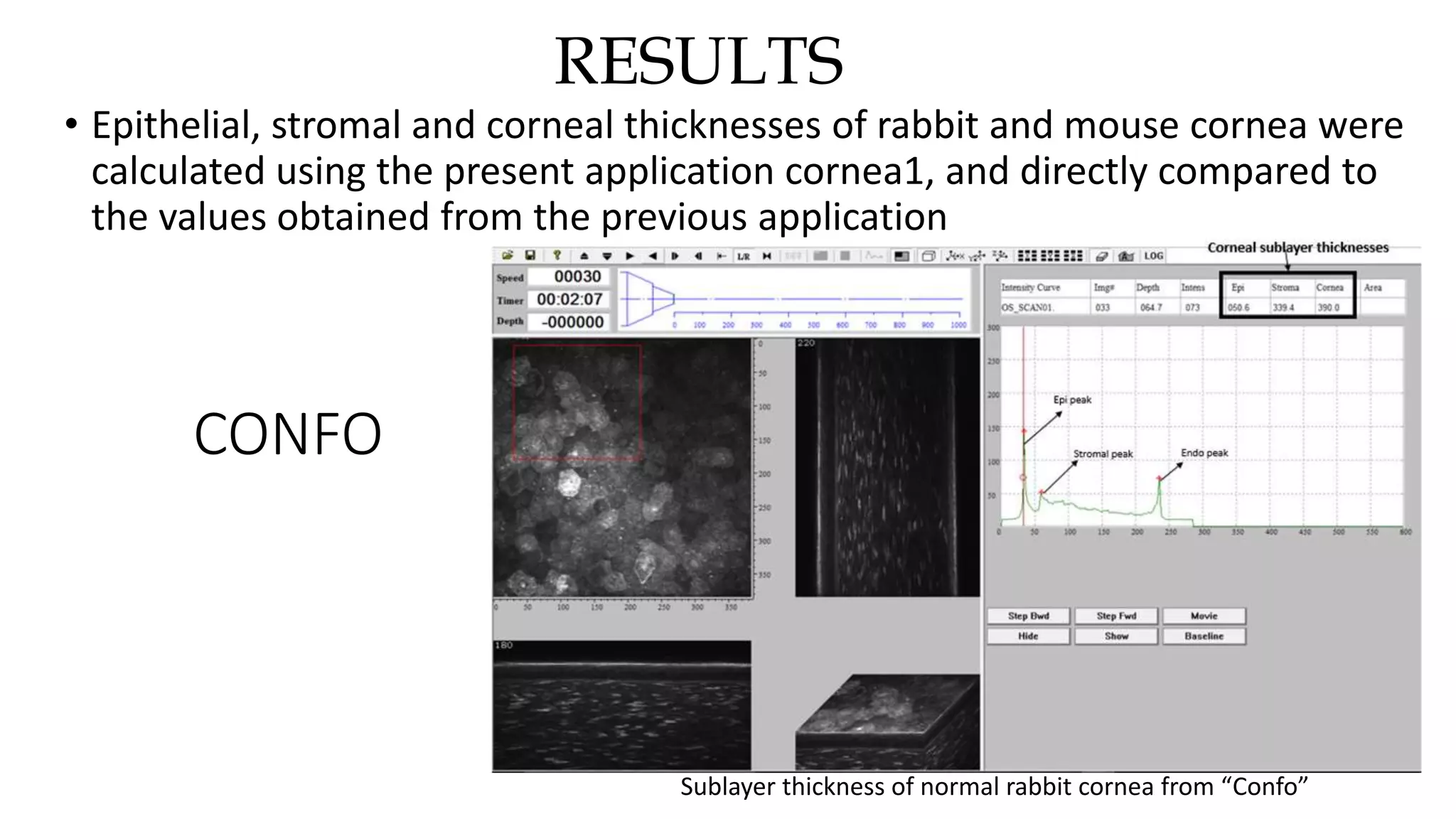
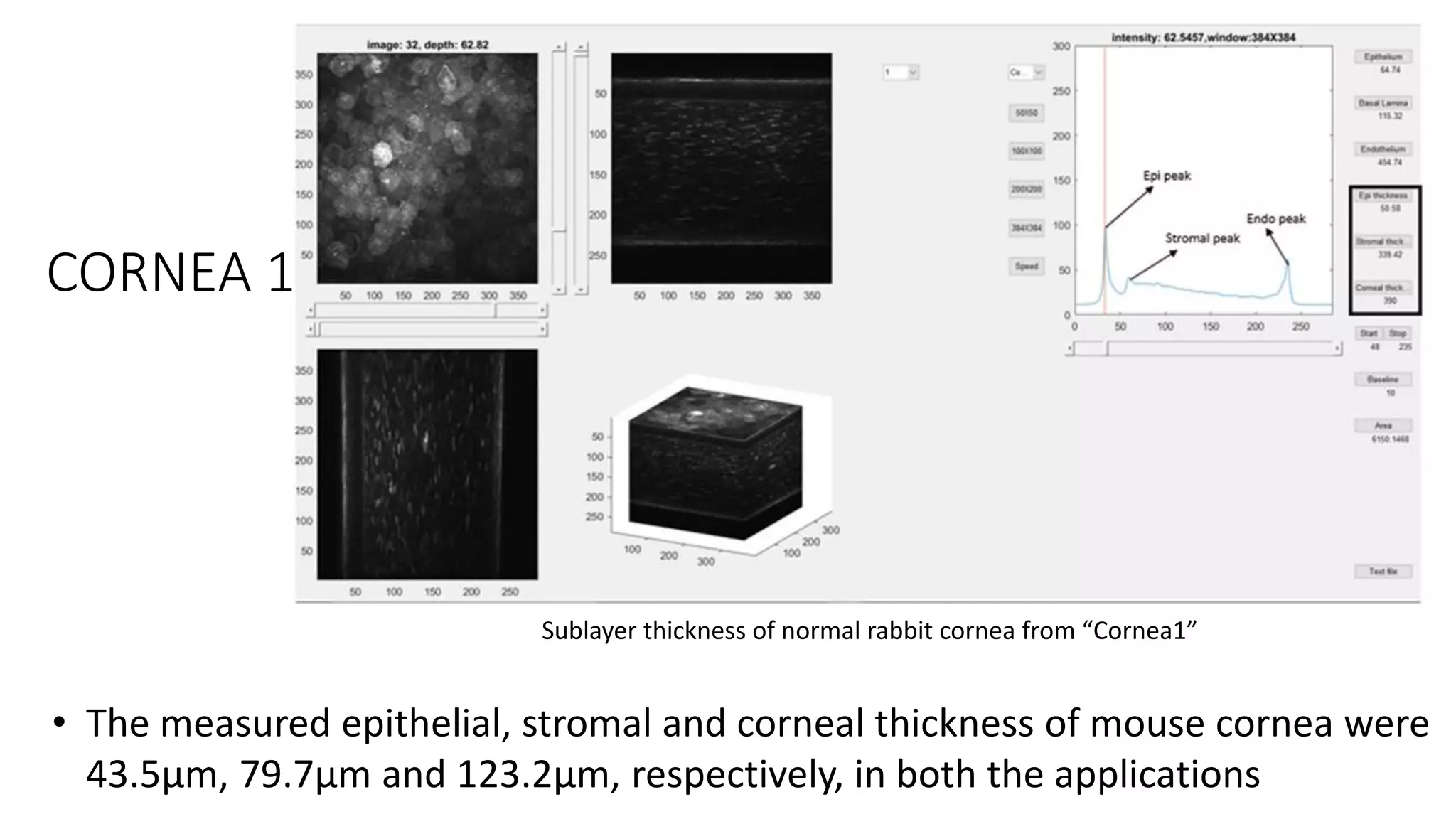
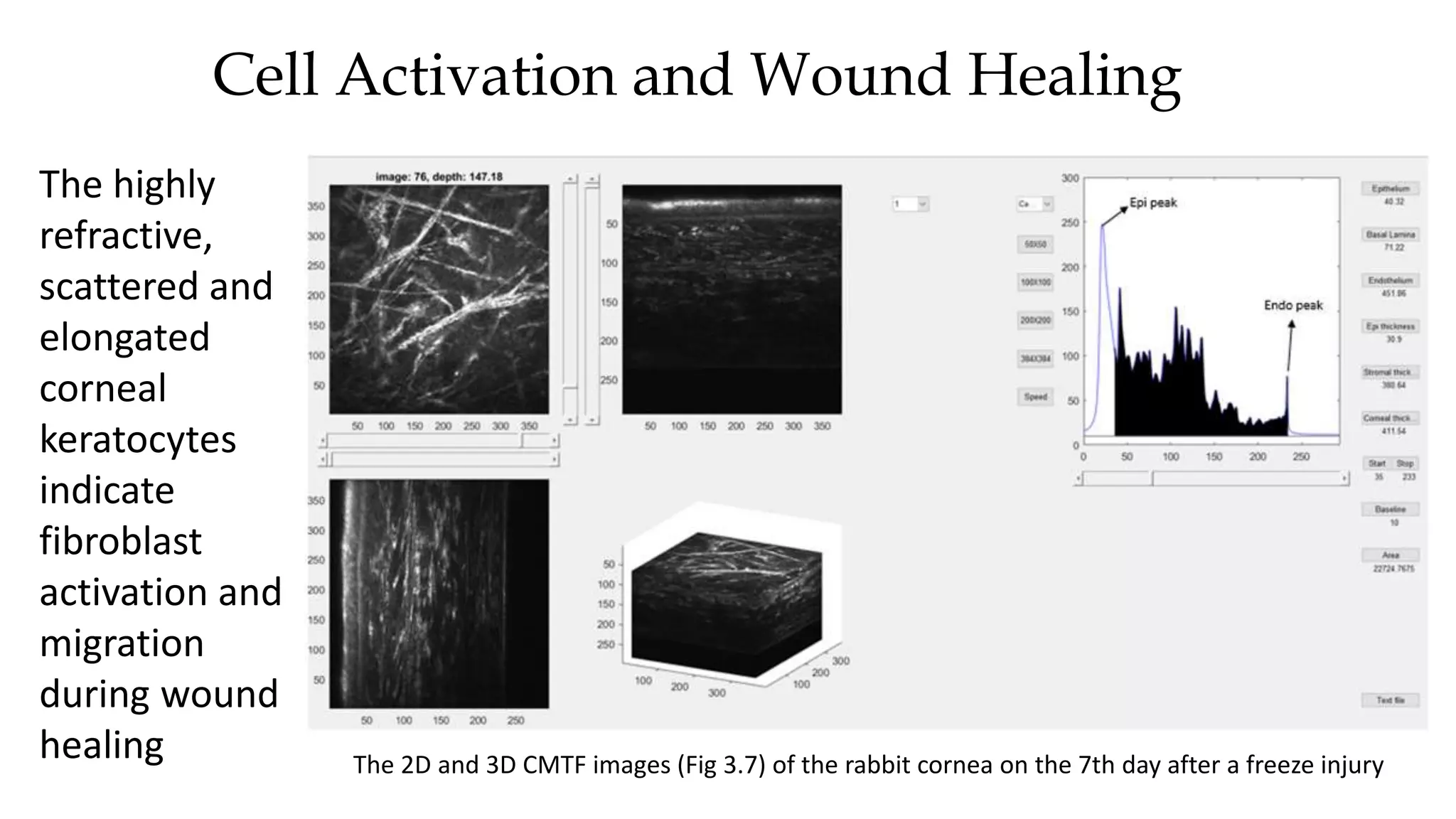
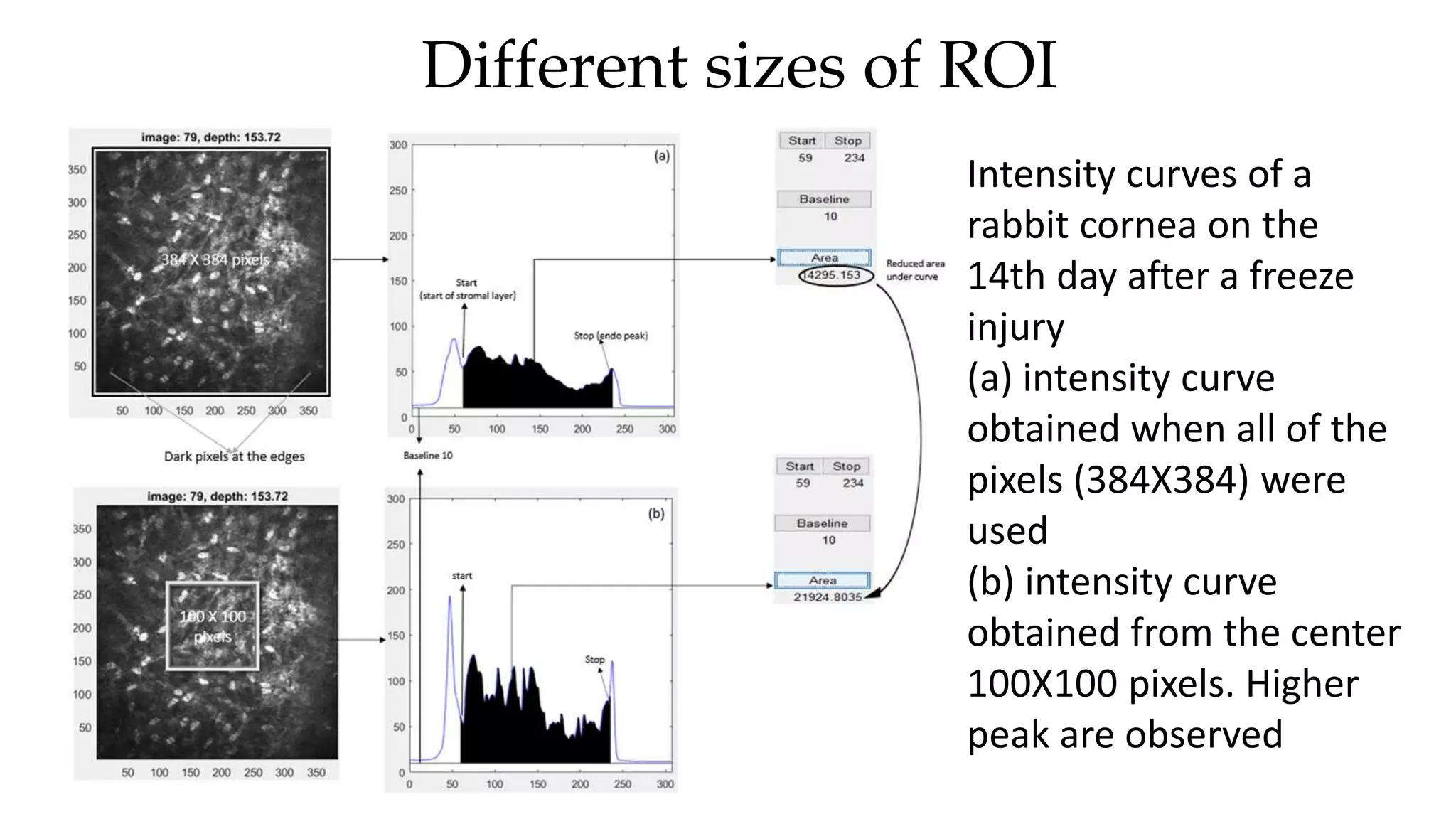
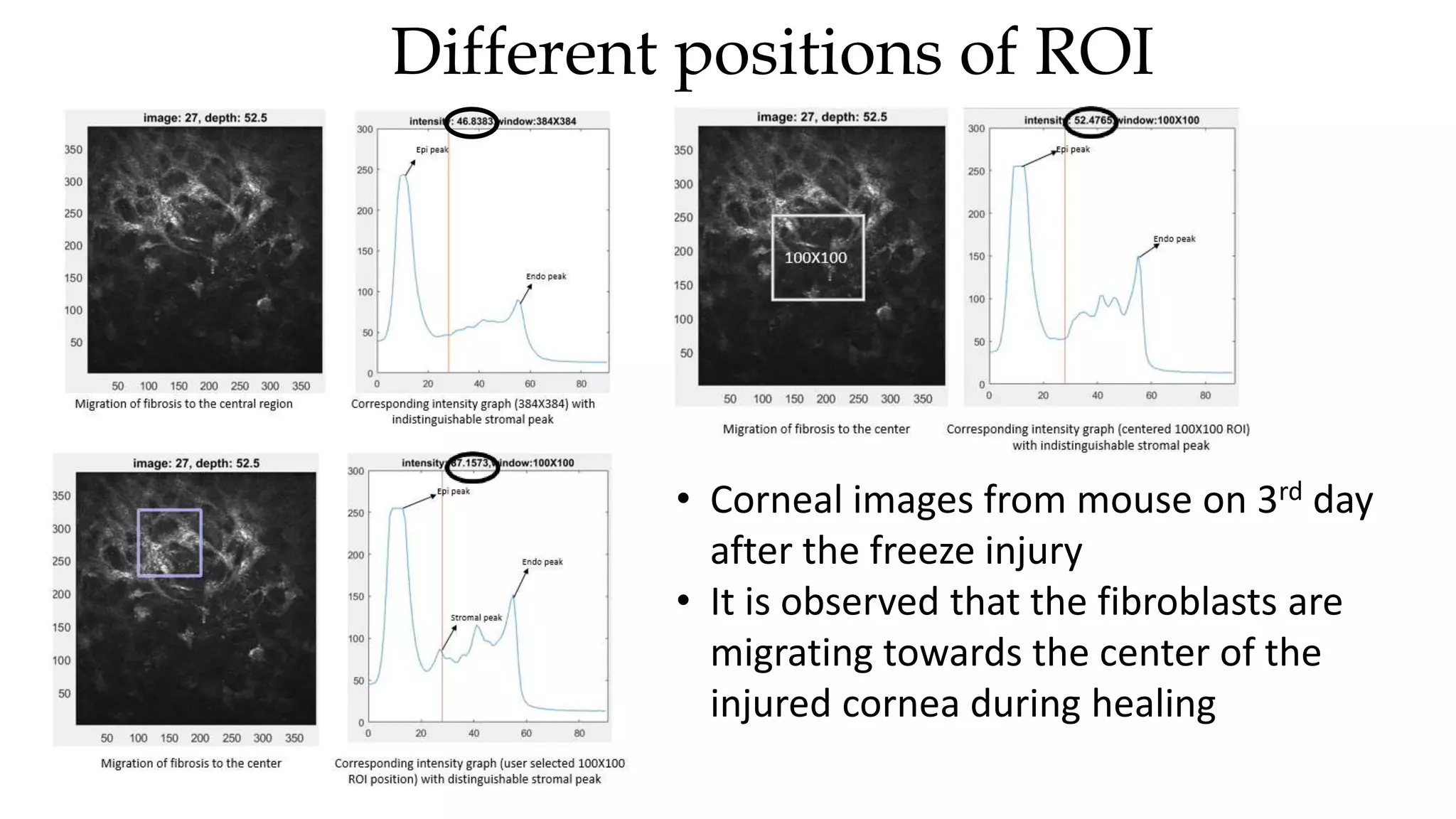
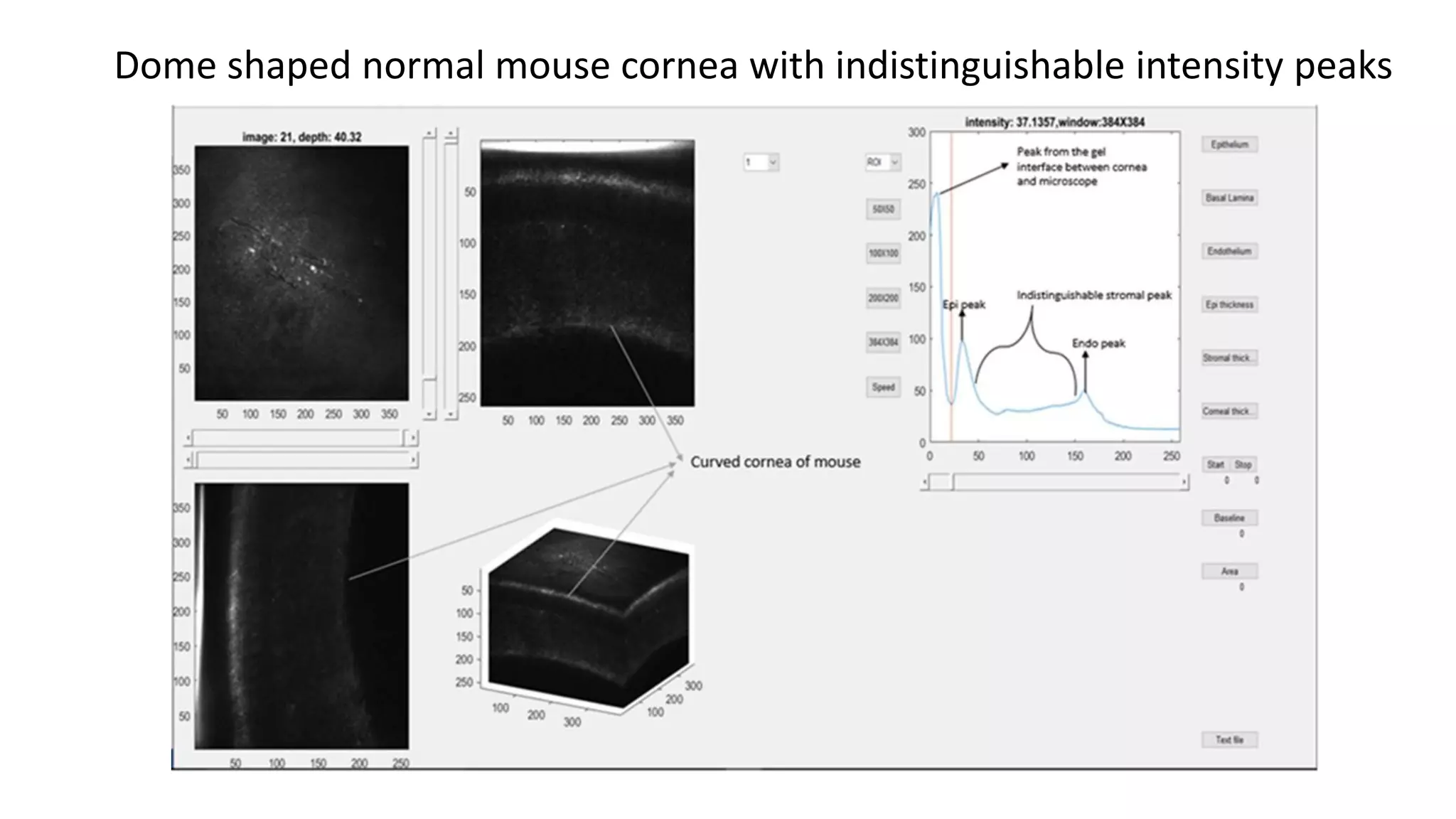
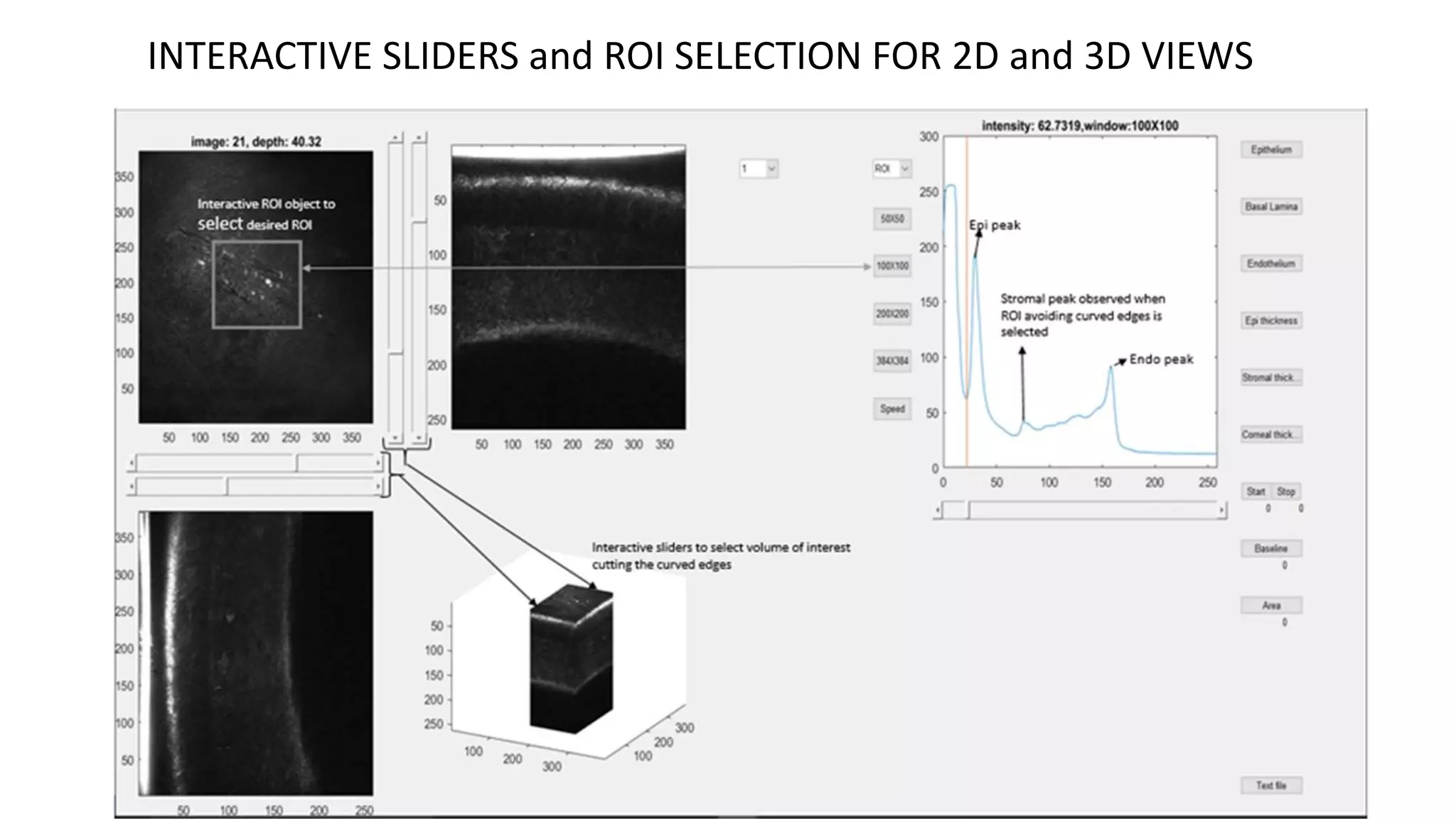
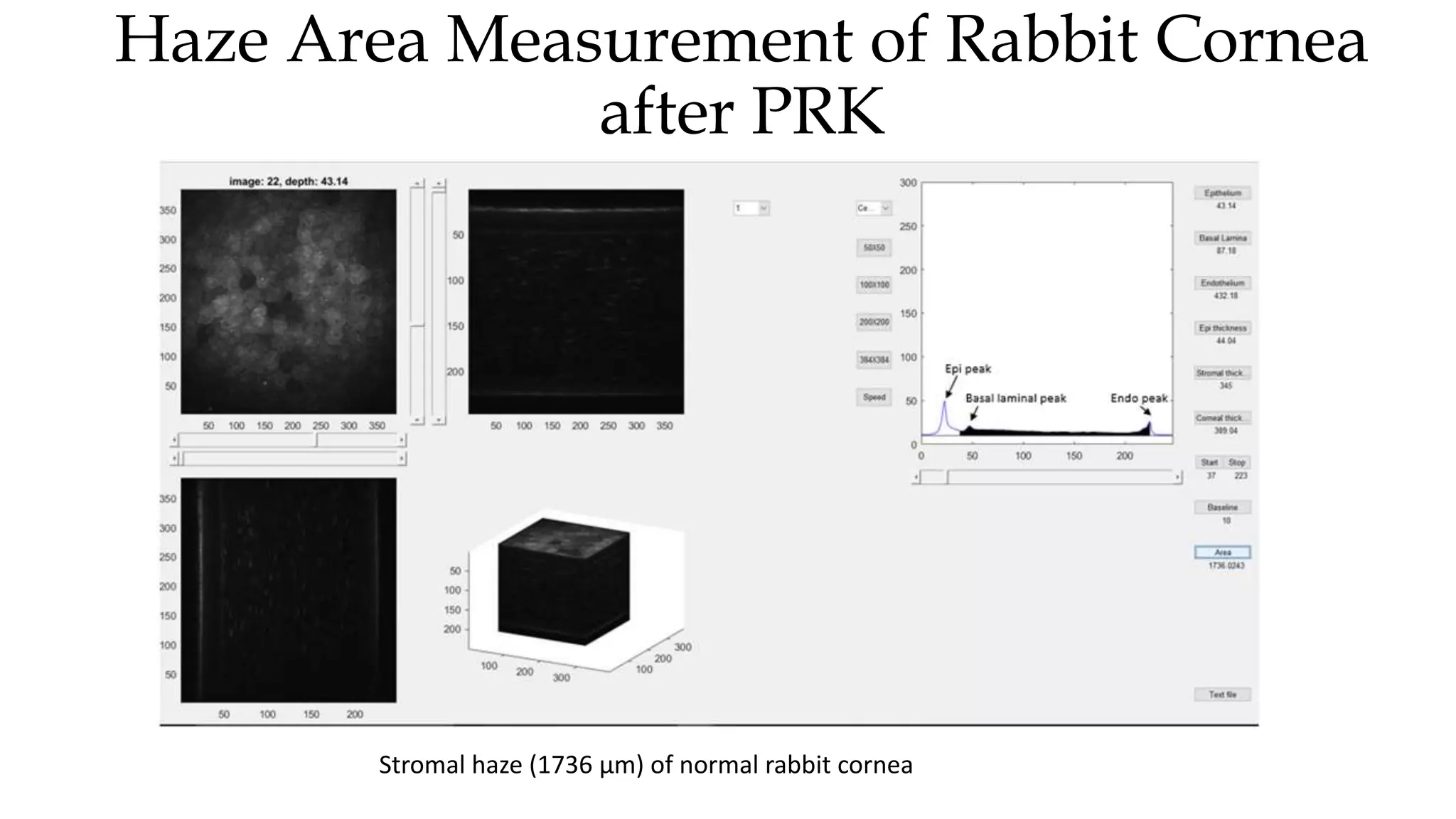
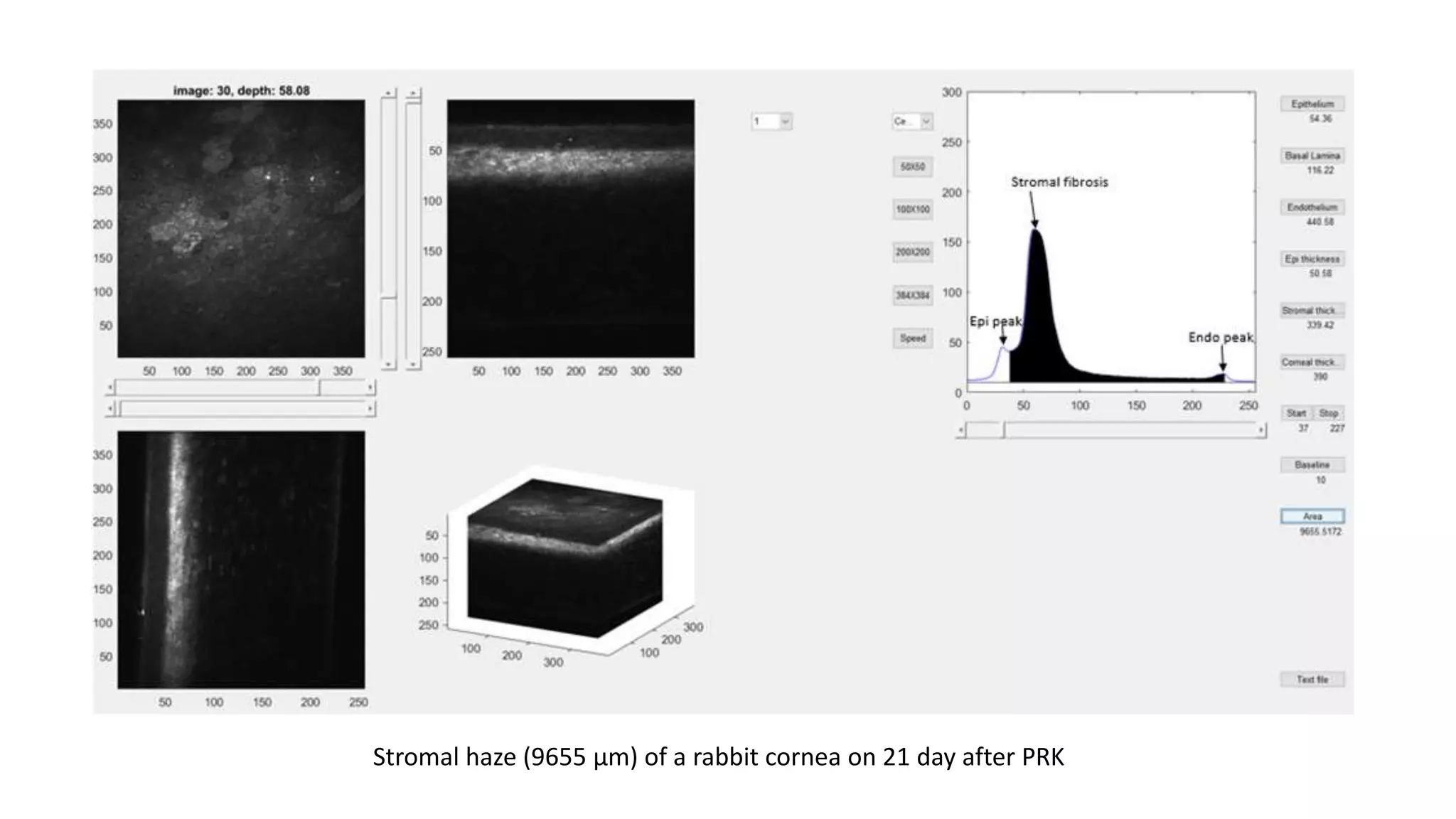
This document describes the development of an interactive analytical tool for quantitative analysis of corneal confocal imaging data. The tool was developed using MATLAB to provide a more user-friendly interface compared to an existing C++-based software. The tool allows the user to interactively select the region of interest for intensity curve calculation from corneal image stacks collected using confocal microscopy. Animal studies using rabbits and mice were conducted to test freeze injury and photorefractive keratectomy models and collect confocal image data for analysis using the new tool.