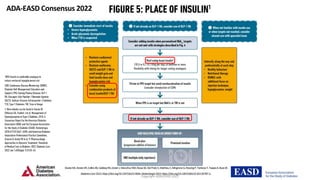
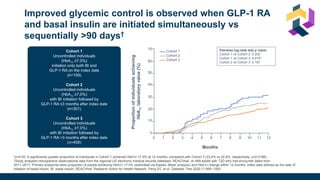
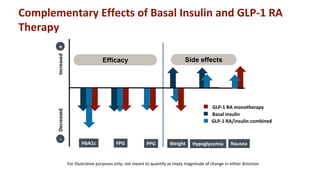
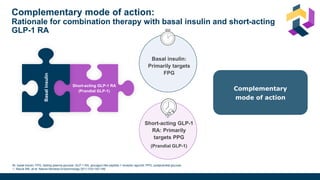
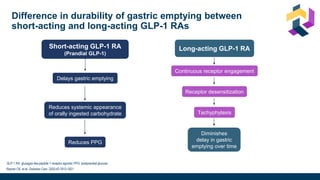
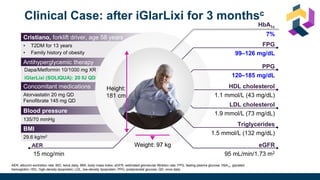
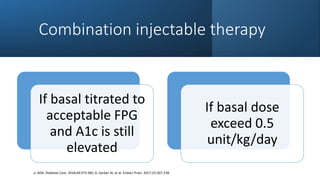
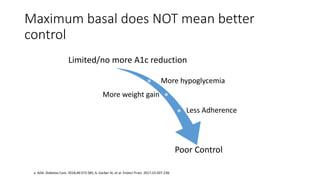
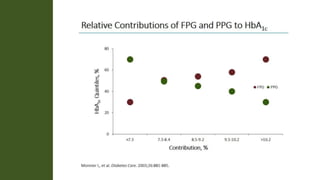
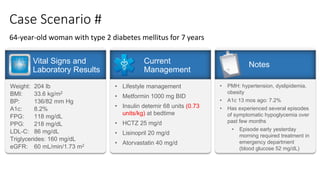
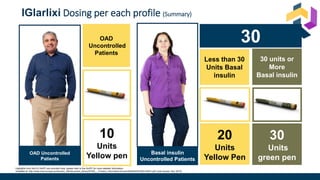
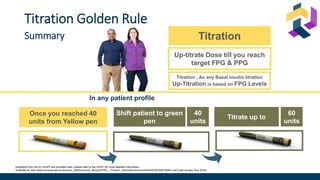
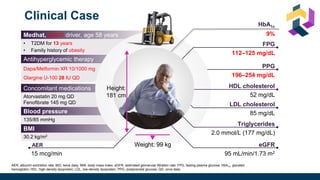
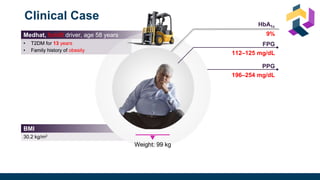
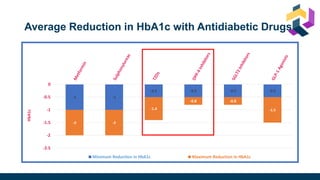
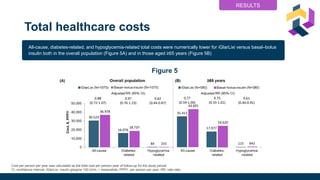
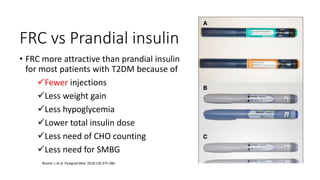
The document discusses various intensification options for insulin therapy in patients with Type 2 Diabetes Mellitus (T2DM), highlighting barriers to therapy upgrade such as clinical inertia, patient fears, and lack of education. It emphasizes a proactive early intervention approach over a stepwise method for better glycemic control. Additionally, it compares the efficacy and safety of using once-daily Iglarlixi against traditional basal-bolus insulin regimens, indicating improved treatment adherence and reduced hypoglycemia rates.











![Study design
A1C, glycated hemoglobin; ED, emergency department; HRU, healthcare resource utilization; iGlarLixi, insulin glargine 100 U/mL + lixisenatide; T2D, type 2 diabetes
Figure 1
• People with T2D aged ≥18 years
at index date
• During baseline period:
• ≥1 fill of basal insulin
• ≥1 valid A1C measurement
• No prior iGlarLixi or premix
insulin or bolus insulin fills
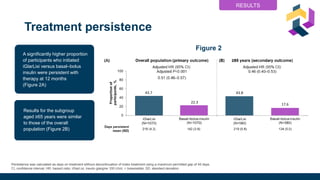
aPrimary outcome (statistical analysis was prespecified for the primary outcome [treatment persistence; overall population] only).
bLaboratory defined; using one count per person per day. cED visits and hospitalizations. dFor inclusion in the A1C analysis participants
had to have a valid baseline and a follow-up A1C value at 12 months; therefore, this analysis was conducted in a small proportion of the
overall population.
METHODS
Newly initiated
iGlarLixi
N = 1082
Newly initiated
basal-bolus
insulin
N = 21,208
N = 1070
N = 1070
6-month baseline treatment
Outcomes at
12 months:
• Persistencea
• Adherence
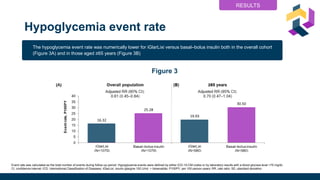
• Hypoglycemiab
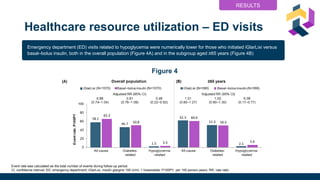
• HRUc
• Costs
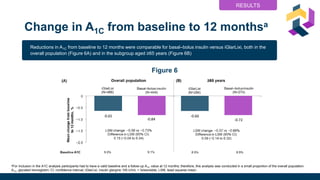
• A1c changed
Assessed in:
• Overall population
• Subgroup aged
> 65 years
Treatment initiation
= index date
Followed from index date for 12 months,
or plan disenrollment or death](https://image.slidesharecdn.com/iglarlixifaqusf-230119000014-1b1303e4/85/Intensification-Options-after-basal-Insulin-Revisited-18-320.jpg)







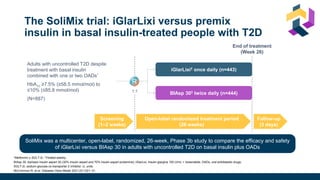
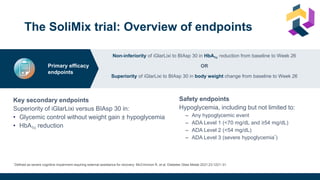
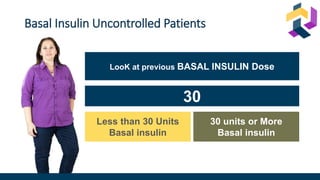
![Once-daily iGlarLixi provided better glycemic control with weight benefit
compared with twice-daily premix BIAsp 30
*Non-inferiority margin 0.3 %.
BIAsp 30, biphasic insulin aspart 30 (30% insulin aspart and 70% insulin aspart protamine); CI, confidence interval; iGlarLixi, a fixed-ratio combination of insulin glargine 100 U/mL and the glucagon-like peptide-1 receptor agonist, lixisenatide;
ITT, intention-to-treat; LS mean, least squares mean; SD, standard deviation; SE, standard error; U, units.
Rosenstock J et al. Diabetes Care 2021;[accepted ahead of publication]
iGlarLixi
(ITT population;
n=443)
BIAsp 30
(ITT population;
n=444)
vs
LS mean ± SE change in HbA1c
from baseline to Week 26, %
−1.3 ± 0.1
−1.1 ± 0.1
Non-inferiority (achieved)
LS mean difference (97.5% CI):
−0.2 (−0.4, −0.1) %; p<0.001
Superiority (achieved)
LS mean difference (95% CI):
−0.2 (−0.4, −0.1) %; p<0.001
LS mean ± SE change in bodyweight
from baseline to Week 26, kg
−0.7 ± 0.2
+1.2 ± 0.2
Superiority (achieved)
LS mean difference (95% CI):
−1.9 (−2.3, −1.4) kg; p<0.001
Subsequent hierarchical testing showed HbA1c reductions were superior with iGlarLixi
versus BIAsp 30 (p=0.001; key secondary endpoint)
iGlarLixi was non-inferior* to BIAsp 30 in
change in HbA1c from baseline to Week 26
HbA1c
iGlarLixi was superior to BIAsp 30 in change in
bodyweight from baseline to Week 26
Mean ± SD HbA1c, % iGlarLixi BIAsp 30
Baseline 8.6 ± 0.7 8.6 ± 0.7
Week 26 7.3 ± 1.1 7.5 ± 1.0
Mean ± SD bodyweight, kg iGlarLixi BIAsp 30
Baseline 80.7 ± 16.6 82.2 ± 18.5
Week 26 80.2 ± 16.6 83.4 ± 19.0](https://image.slidesharecdn.com/iglarlixifaqusf-230119000014-1b1303e4/85/Intensification-Options-after-basal-Insulin-Revisited-35-320.jpg)
![HbA1c target achievement without weight gain and without
hypoglycemia was greater with iGlarLixi vs BIAsp 30
*Not included in the multiple testing procedure. †Hierarchical analysis adjusted for multiplicity. ITT, intent-to-treat; OR, odds ratio.
Rosenstock J, et al. Diabetes Care 2021;dc210393 [online ahead of print].
27.5 (n=122)
12.4 (n=55)
19.4 (n=86)
7.0
(n=31)
1.65 (1.25, 2.19)*
0 10 20 30 40 50
Percentage of participants reaching target (%)
HbA1c <7 %
(Exploratory analysis*)
HbA1c <7 % without
weight gain
(Key secondary endpoint†)
HbA1c <7 % without
weight gain and
without hypoglycemia
(Key secondary endpoint†)
OR (95% CI)
iGlarLixi
(ITT population; N=443)
BIAsp 30
(ITT population; N=444)
42.2 (n=187)
31.8 (n=141)
3.40 (2.19, 5.28)†
p<0.001
2.83 (1.98, 4.04)†
p<0.001](https://image.slidesharecdn.com/iglarlixifaqusf-230119000014-1b1303e4/85/Intensification-Options-after-basal-Insulin-Revisited-36-320.jpg)