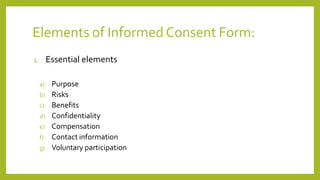
The document outlines the key elements that must be included in an informed consent process and document according to various guidelines. It discusses the definition of informed consent, the process of obtaining consent which includes provision of information, comprehension, and voluntary participation. It also describes the key components that must be included in an informed consent form such as the purpose of the study, risks and benefits, confidentiality, compensation, and voluntary participation. Reference guidelines from ICMR, ICH GCP, FDA and CFR are provided on the required elements of informed consent.