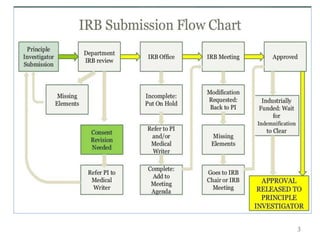
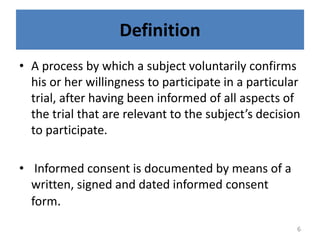
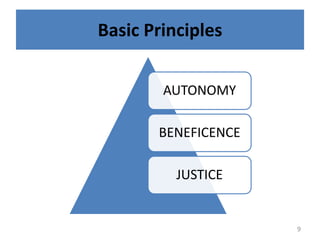
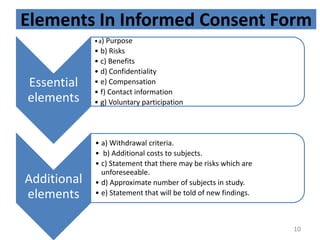
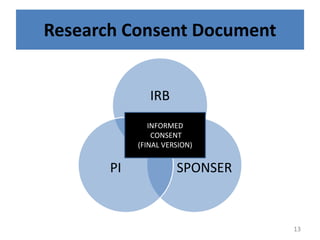
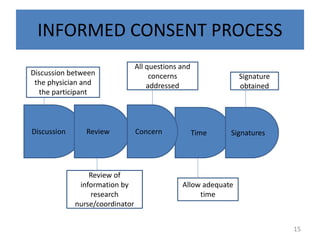
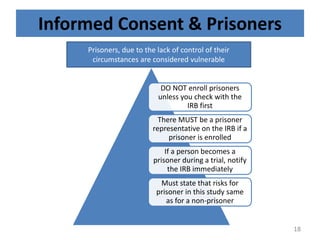
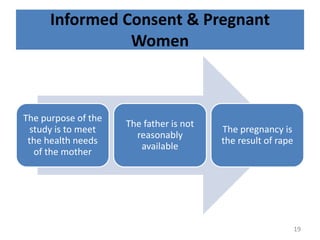
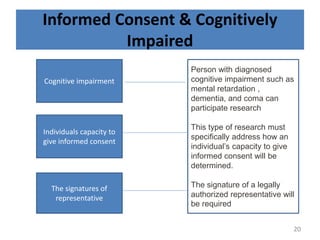
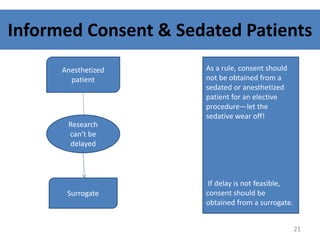
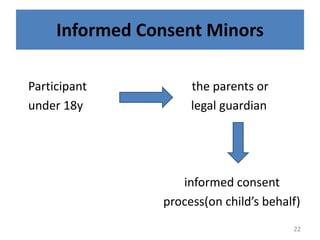
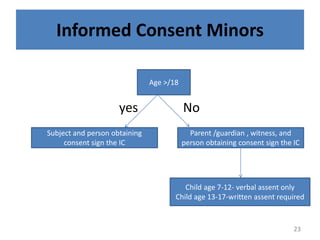
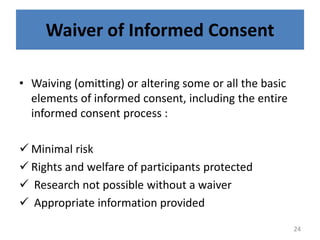
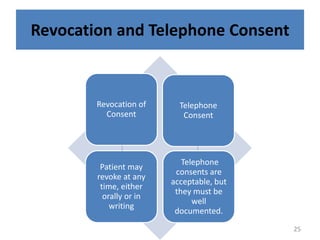
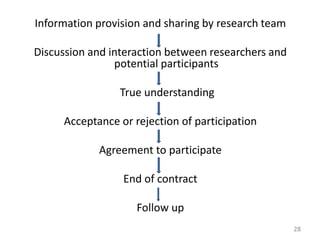
The document discusses the informed consent process for clinical trials. It defines informed consent as a voluntary agreement to participate in research after being informed of all aspects relevant to the decision. The summary elements of informed consent include understanding the purpose and risks of the study, making a voluntary decision about participation, and being informed of alternative options. An effective informed consent process involves clear communication between researchers and participants throughout the study.