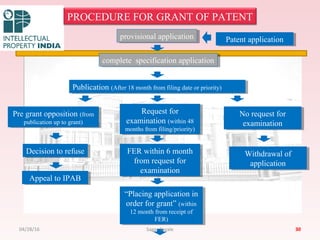
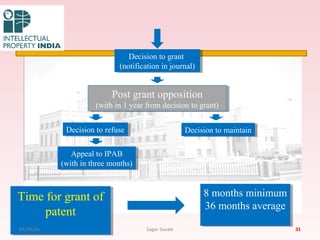
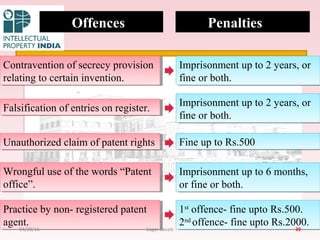
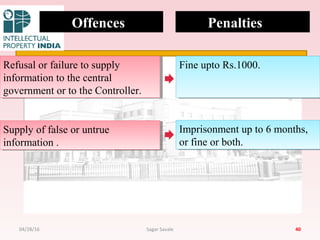
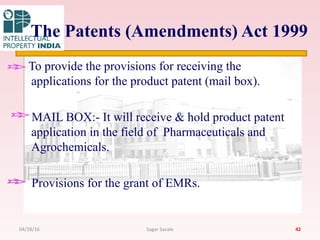
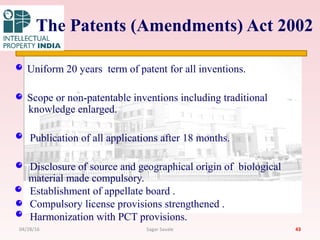
The Indian Patent Act of 1970 outlines the framework for patent protection, including definitions, application procedures, types of patents, and amendments over the years. It highlights key changes like the introduction of product patents and measures to enhance intellectual property rights in India in accordance with international treaties. The act also addresses issues of patent infringement, rights of patentees, and penalties for violations.











![Terms & Definition
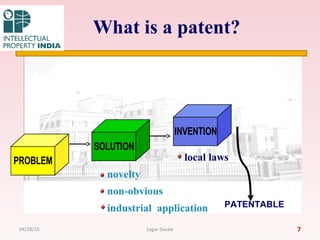
INVENTION – [ Acc. To sec 2 (1)(j) ]
Invention means a new product or process involving an
inventive step & capable of industrial application.
or
It is a conception of new ideas.
INNOVATION – It is application adoption of the idea.
e.g. it is the process that moves the idea into market place.
1604/28/16 Sagar Savale](https://image.slidesharecdn.com/indianpatentact1970-160428053830/85/Indian-patent-act-1970-16-320.jpg)