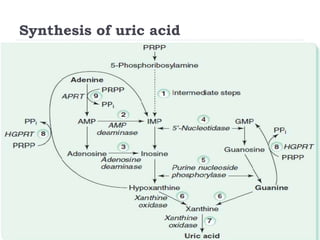
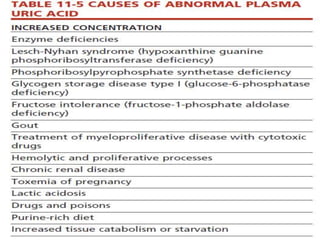
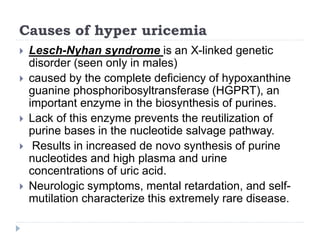
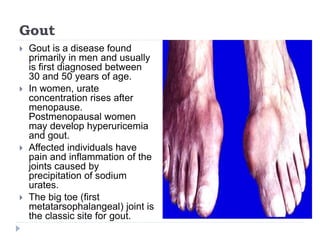
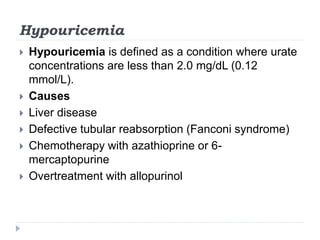
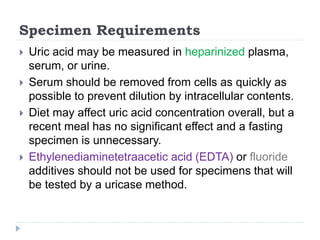
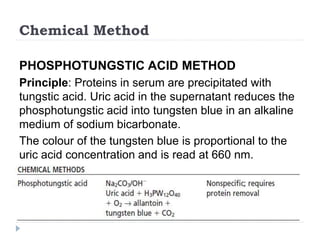
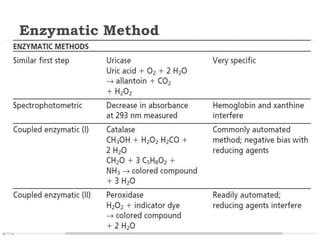
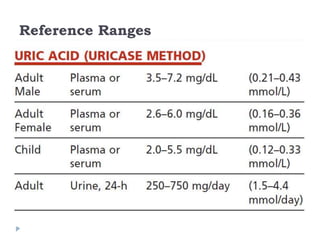
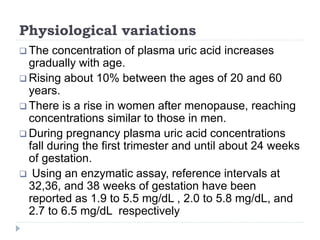
This document summarizes information about hyperuricemia and uric acid. It discusses that uric acid is produced from the breakdown of purines and defines hyperuricemia. The document outlines the physiology of uric acid production and excretion by the kidneys. It describes some causes of high uric acid levels like Lesch-Nyhan syndrome and gout. The document also discusses hypouricemia and mentions several methods for measuring uric acid levels like chemical, enzymatic, IDMS and HPLC methods. It provides reference ranges and notes some physiological variations in uric acid levels.