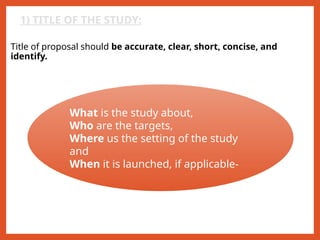
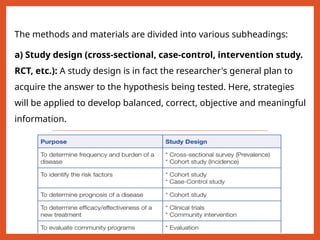
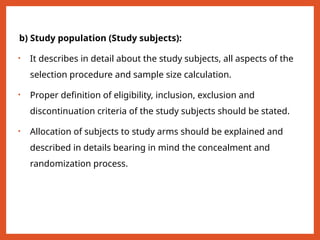
The document outlines the importance of writing research protocols in clinical studies, defining a protocol as a document that sets rules and outlines the study plan to safeguard participants' health while addressing specific research questions. It details the aims, benefits, key components, and methodological considerations necessary for crafting a comprehensive protocol, including elements like study objectives, hypothesis formulation, data management, and ethical approvals. A well-written protocol is essential for guiding research, evaluating literature, and ensuring the study's design results in reliable and valid outcomes.