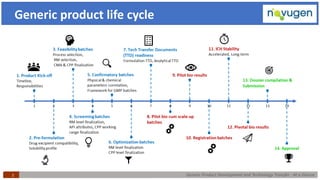
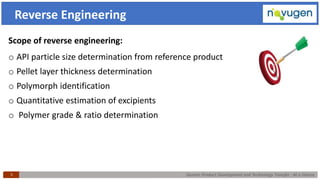
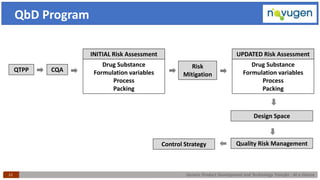
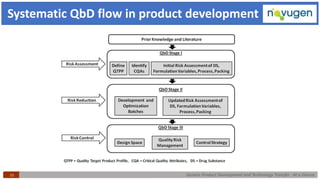
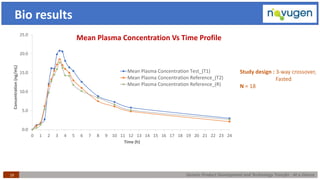
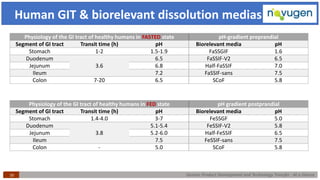
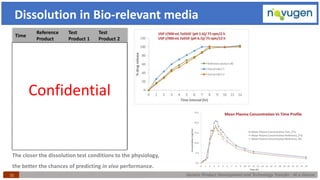
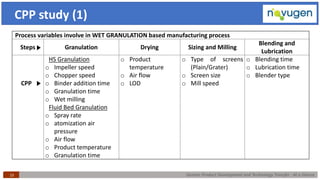
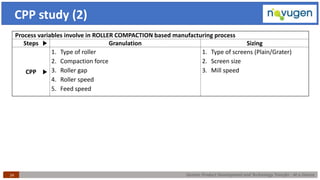
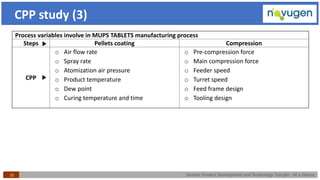
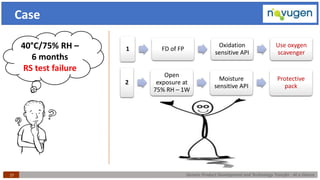
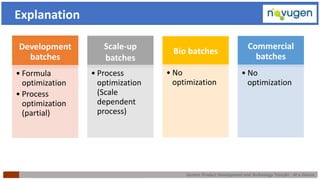
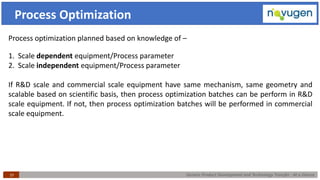
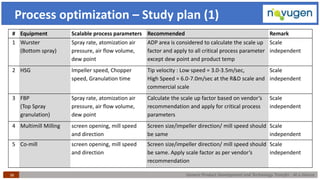
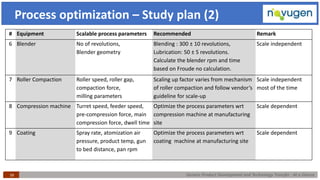
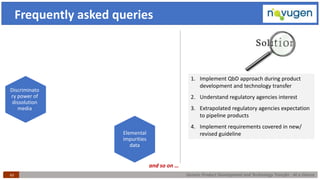
The document outlines the challenges and remedies in generic product development and technology transfer, highlighting significant issues such as difficult product formulation and regulatory queries. It emphasizes the application of Quality by Design (QbD) principles, reverse engineering techniques, and specific case studies related to drug formulation and bioavailability. Through systematic approaches and thorough understanding of product and process, the text calls for efficient regulatory compliance and improved product quality assurance.