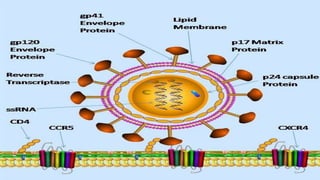
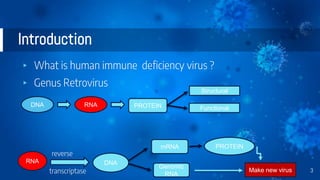
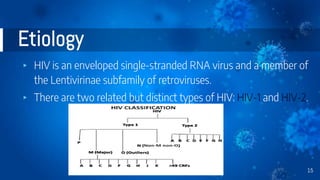
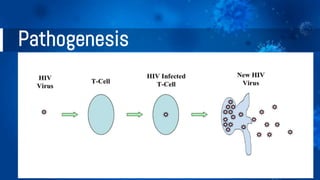
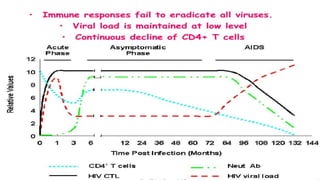
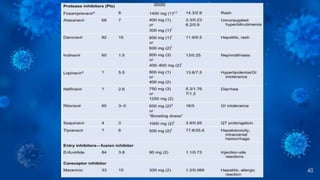
The document provides a comprehensive overview of human immunodeficiency virus (HIV), detailing its classification, epidemiology, etiology, pathogenesis, diagnosis, clinical presentation, and management strategies. It emphasizes the impact of HIV on the immune system, the global prevalence of the virus, and treatment protocols, including antiretroviral therapy and post-exposure prophylaxis. Furthermore, it outlines the clinical management of opportunistic infections related to HIV, such as pneumocystis carinii pneumonia.






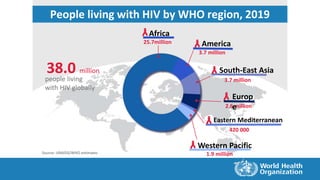
![Source: UNAIDS/WHO estimates
0.7millionHIV-related deaths
[0.5 million –1.0 million]
1.7million
people newly infected
[1.2 million – 2.2 million]
38.0million
people living with HIV
[31.6 million – 44.5 million]
2019
Summary of the global HIV epidemic, 2019](https://image.slidesharecdn.com/hivinfection-201205060611/85/Hiv-infection-9-320.jpg)
![Summary of the global HIV epidemic, 2019
38.0 million
[31.6 million – 44.5 million]
36.2 million
[30.2 million – 42.5 million]
19.2 million
[16.4 million – 22.2 million]
17.0 million
[13.8 million – 20.4 million]
1.8 million
[1.3 million – 2.2 million]
1.7 million
[1.2 million – 2.2 million]
150 000
[94 000 – 240 000]
1.5 million
[1.1 million – 2.0 million]
690 000
[500 000 – 970 000 million]
600 000
[430 000 – 840 000]
95 000
[61 000 – 150 000]
Source: UNAIDS/WHO estimates
People living with
HIV in 2019
People newly
infected with HIV in
2019
HIV-related
deaths 2019
870 000
630 000 – 1.2 million]
790 000
590 000 – 1.1 million]
390 000
[280 000 – 560 000]
300 000
[220 000 – 420 000]](https://image.slidesharecdn.com/hivinfection-201205060611/85/Hiv-infection-10-320.jpg)




























![Clinical presentation of (Pneumocystis jiroveci)
▸ Symptoms fever and dyspnea
▸ clinical signs are tachypnea, with or without rales or rhonchi, and a
nonproductive or mildly productive cough.
▸ Arterial blood gases
▸ may show minimal hypoxia (partial pressure of oxygen [PaO2] 80 to 95
mm Hg
▸ [10.6–12.6 kPa]) but in more advanced disease may be markedly abnormal.
50](https://image.slidesharecdn.com/hivinfection-201205060611/85/Hiv-infection-50-320.jpg)