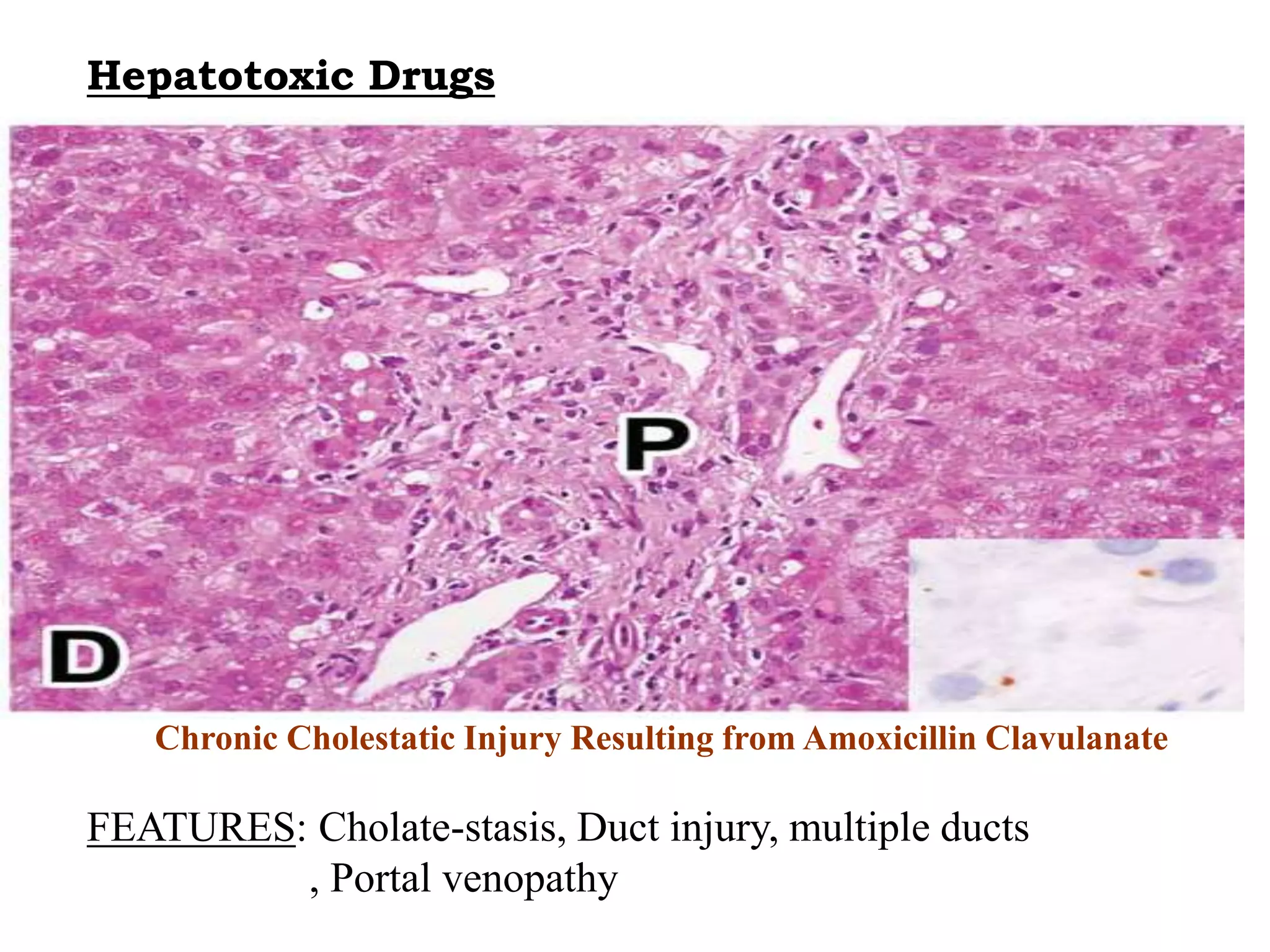
This document discusses hepatotoxic drugs and their mechanisms of causing liver damage. It begins by classifying hepatotoxic drugs into intrinsic, idiosyncratic, and chronic categories. It then describes various mechanisms by which drugs can damage the liver, including by forming reactive metabolites, depleting glutathione, and interfering with mitochondrial functions. Specific hepatotoxic drugs are listed for different drug classes. Methods for evaluating hepatotoxicity both in vivo and in vitro are also presented.