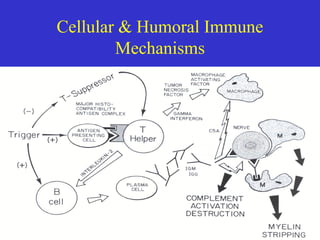
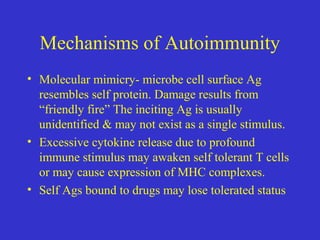
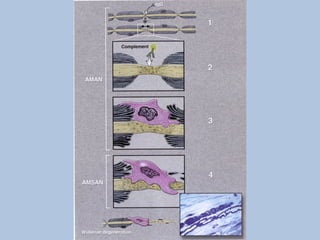
This document describes a case of Guillain-Barré syndrome (GBS) in a 40-year-old woman. She developed nausea, diarrhea, and muscle aches after her son was diagnosed with rotavirus. Later she experienced numbness and tingling in her legs that progressed to weakness. Testing showed findings consistent with GBS including abnormal electromyography and absent reflexes. She was treated with intravenous immunoglobulin, showed some improvement, and was discharged.