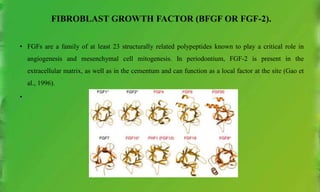
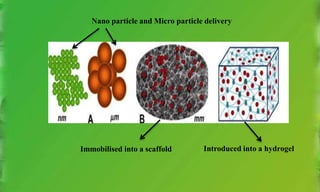
This document discusses various growth factors and their roles in periodontal tissues and regeneration. It defines growth factors and their modes of action. It then classifies and describes several important growth factors in detail, including PDGF, TGFs, BMPs, CTGF, FGFs. It discusses the use of growth factors like PDGF and BMPs in therapies and delivery methods.















![THE BONE MORPHOGENETIC FAMILY
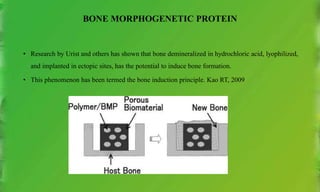
• The BMPs were discovered based on their presence in purified bone inductive extracts derived from bone.
• An extensive purification (more than 300,000-fold) was required to provide protein of sufficient purity. This
suggests that the osteoinductive proteins are minor components of bone matrix, and present at lower levels than
many other growth factors.
• BMP”s have been isolated from bovine and human sources out of which bone morphogenic protein-2
(osteopontin-2 [OP-2]), bone morphogenic protein-3 - periodontal Regeneration. Massagué J 1998
• The hallmark property of BMP is the differentiation factor. BMP will differentiate an undifferentiated
mesenchymal cell into an osteoblast.
• In contrast, PDGF is a chemotactic and mitogenic factor for osteoblast like precursors. Okano T, 1990](https://image.slidesharecdn.com/growthfactors-210210064027/85/Growth-factors-26-320.jpg)