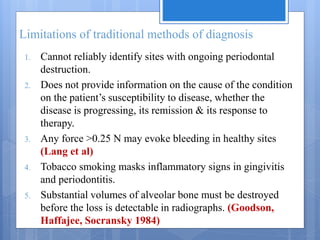
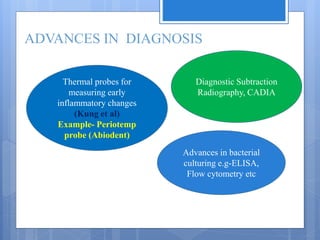
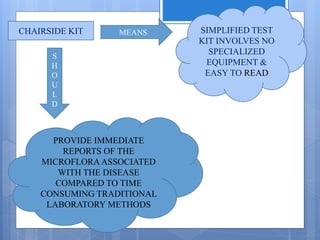
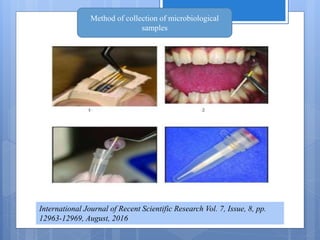
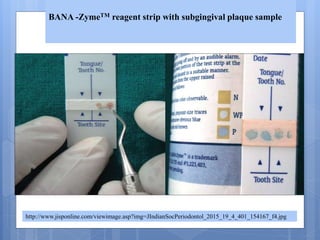
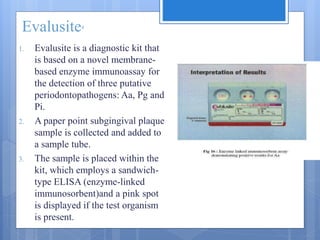
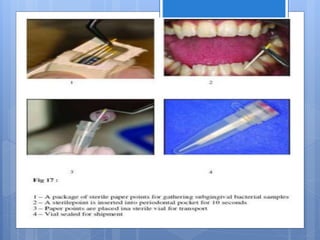
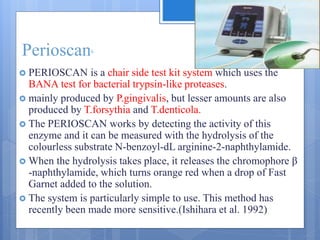
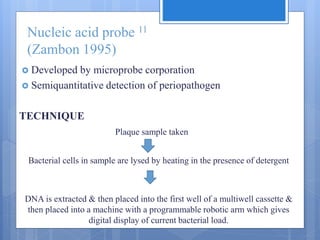
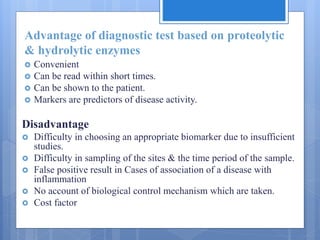
The document discusses various chairside diagnostic aids that can be used in periodontal examination. It outlines the limitations of traditional diagnostic methods like clinical and radiographic evaluation. It then describes several advanced diagnostic aids like thermal probes, subtraction radiography. The rationale for developing chairside diagnostic kits is provided which allow immediate reports without specialized equipment. Examples of microbiological, genetic and biochemical chairside test kits are explained in detail, covering their methodology and biomarkers analyzed. Newer diagnostic tests still under development are also mentioned.