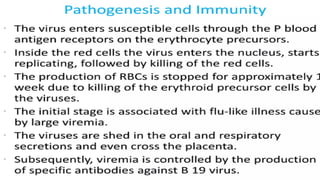
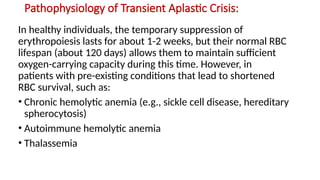
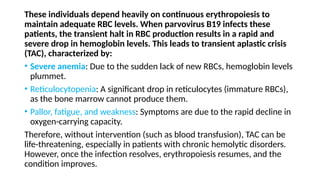
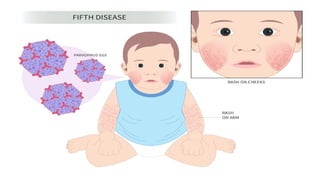
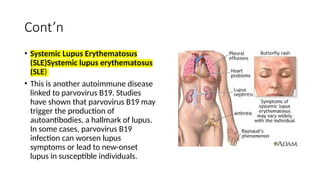
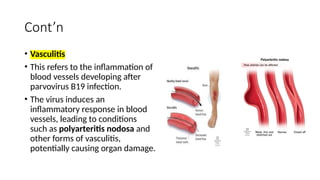
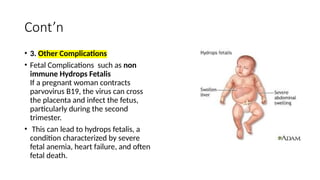
The document discusses the pathogenesis of parvovirus B19, which primarily targets erythroid progenitor cells and leads to conditions like aplastic anemia and erythema infectiosum. The immune response is crucial for disease control but also contributes to clinical symptoms, such as joint pain in adults and severe anemia in individuals with chronic hemolytic disorders. Additionally, the document highlights risks associated with maternal infection during pregnancy, potential long-term complications like chronic anemia and autoimmune conditions, and the significant implications for immunocompromised patients.