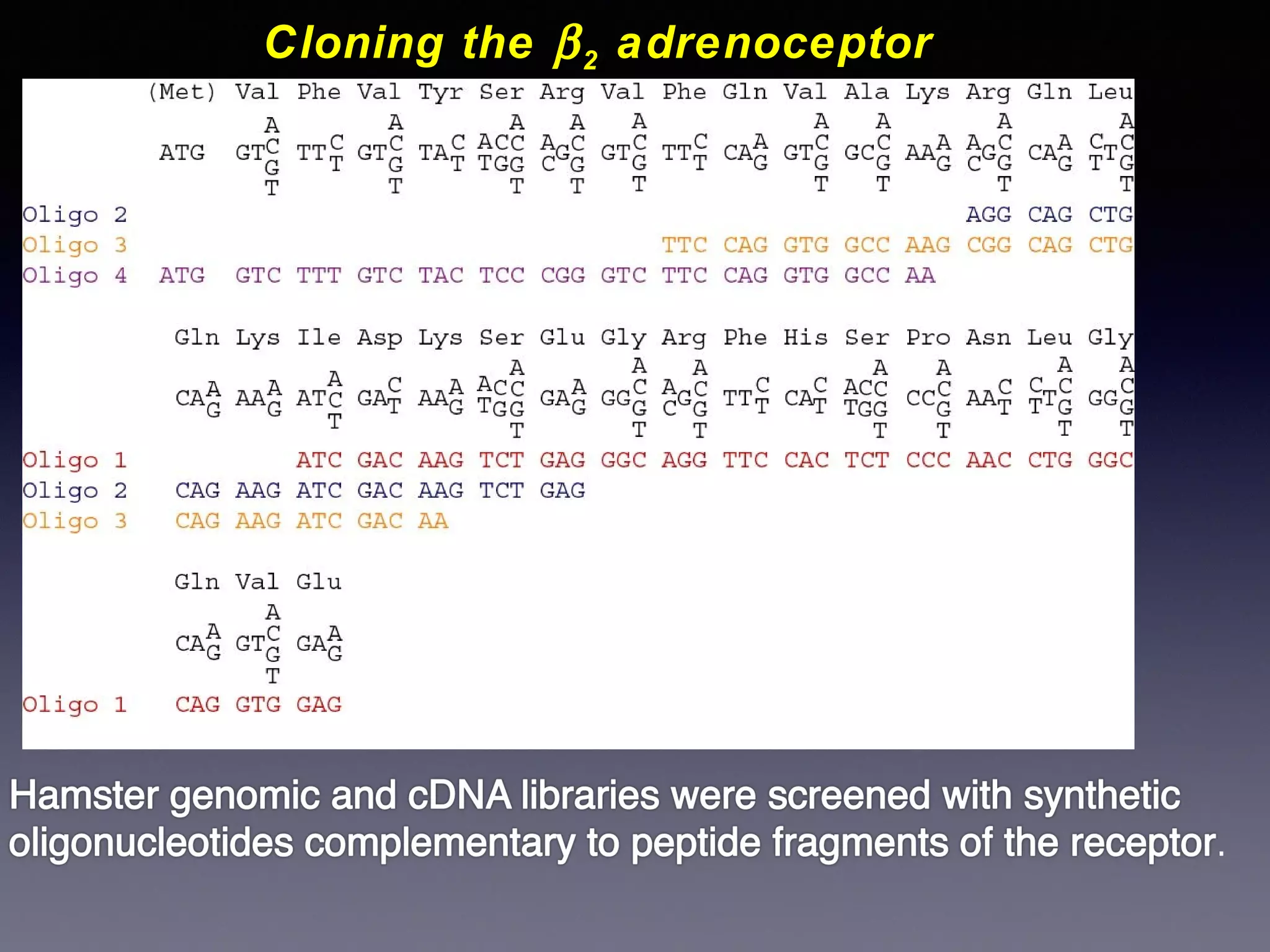
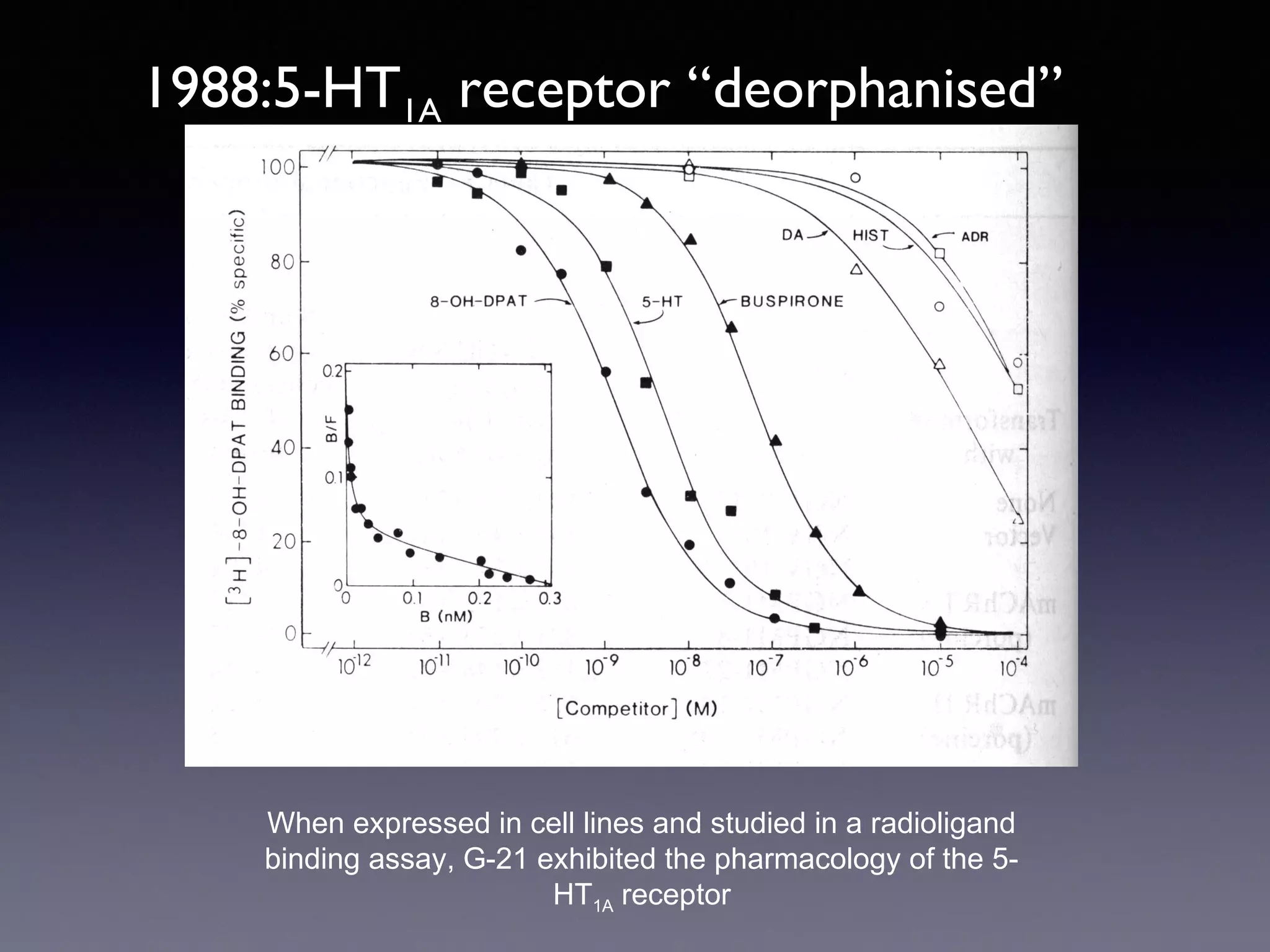
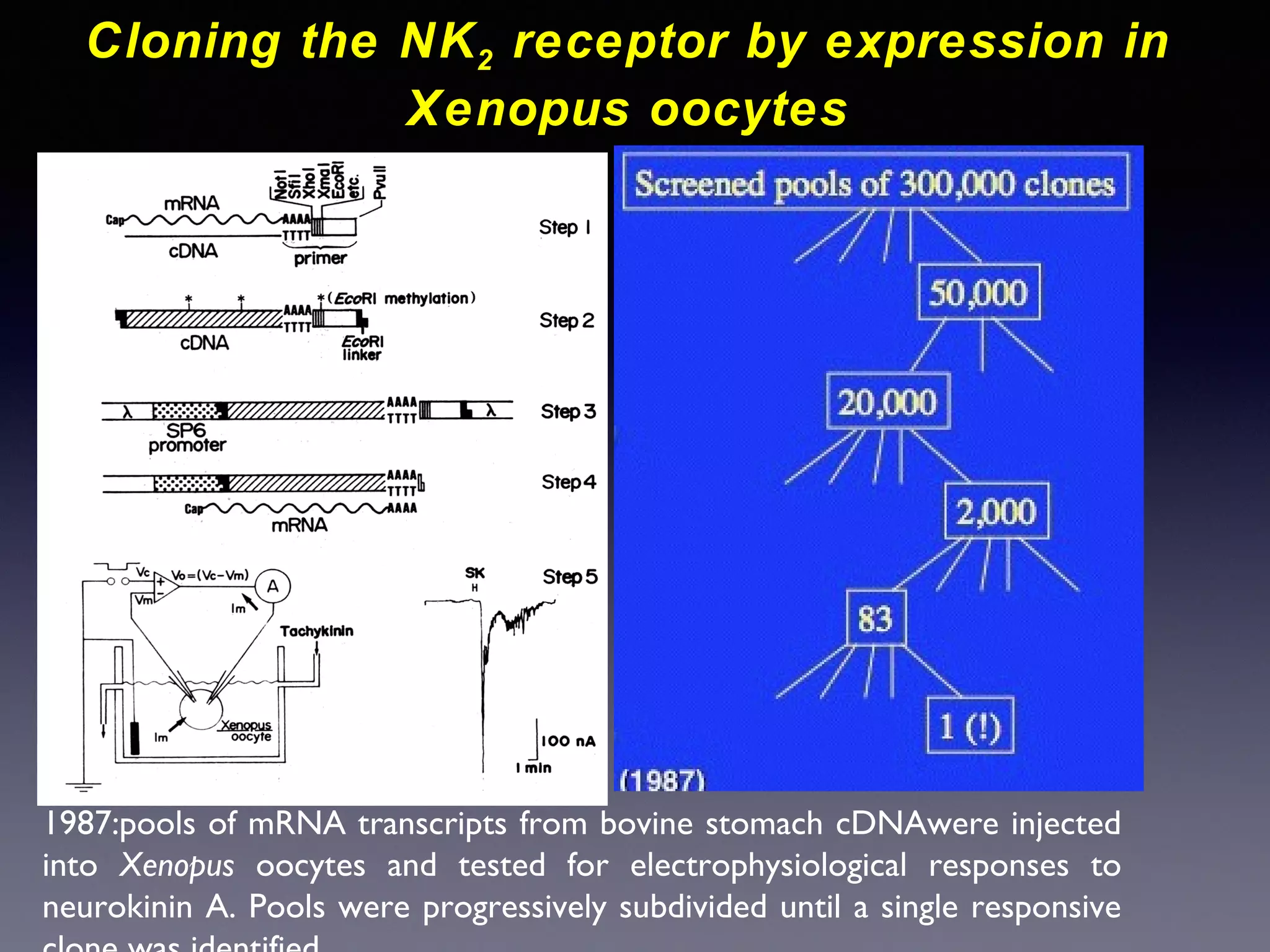
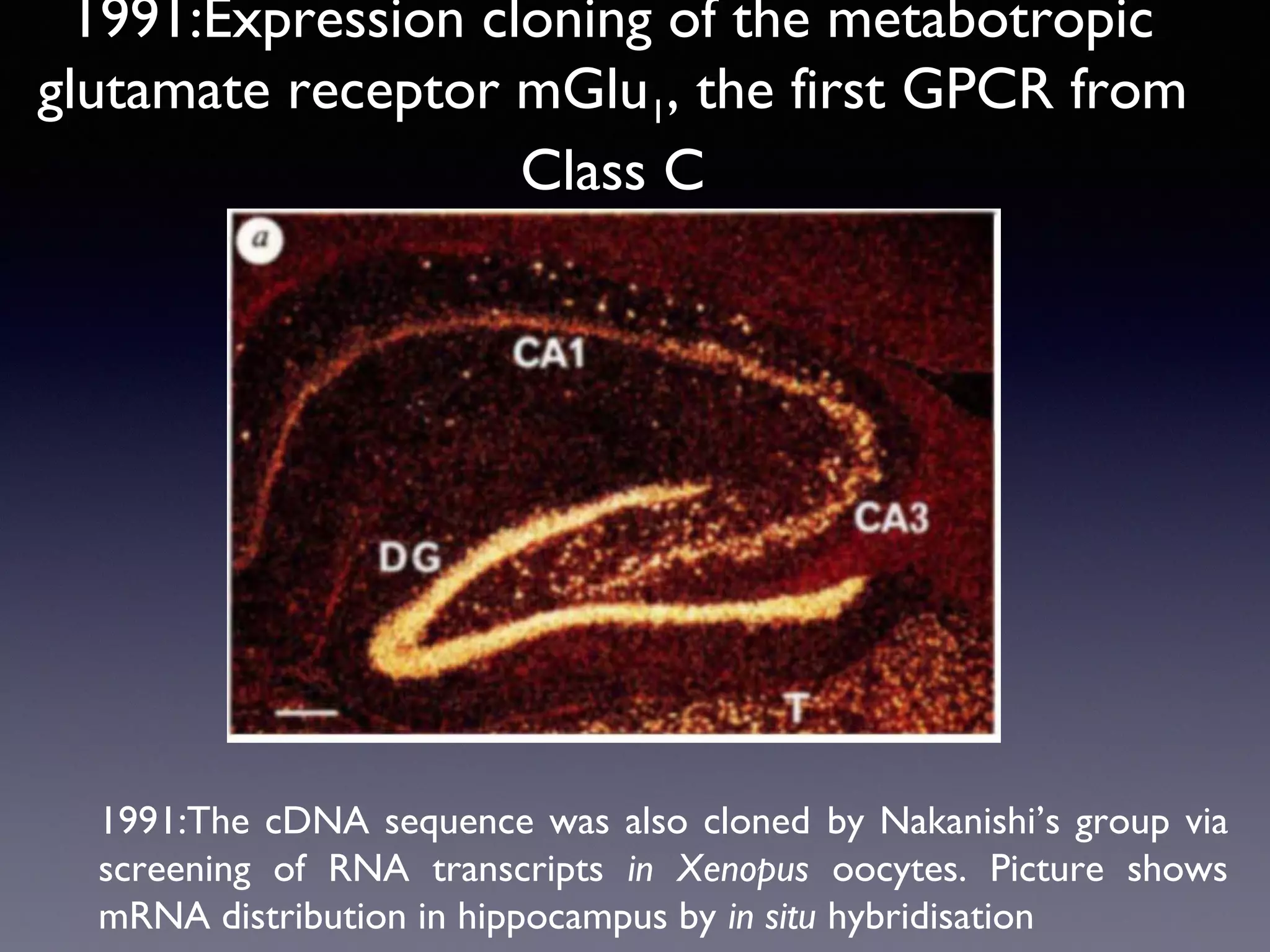
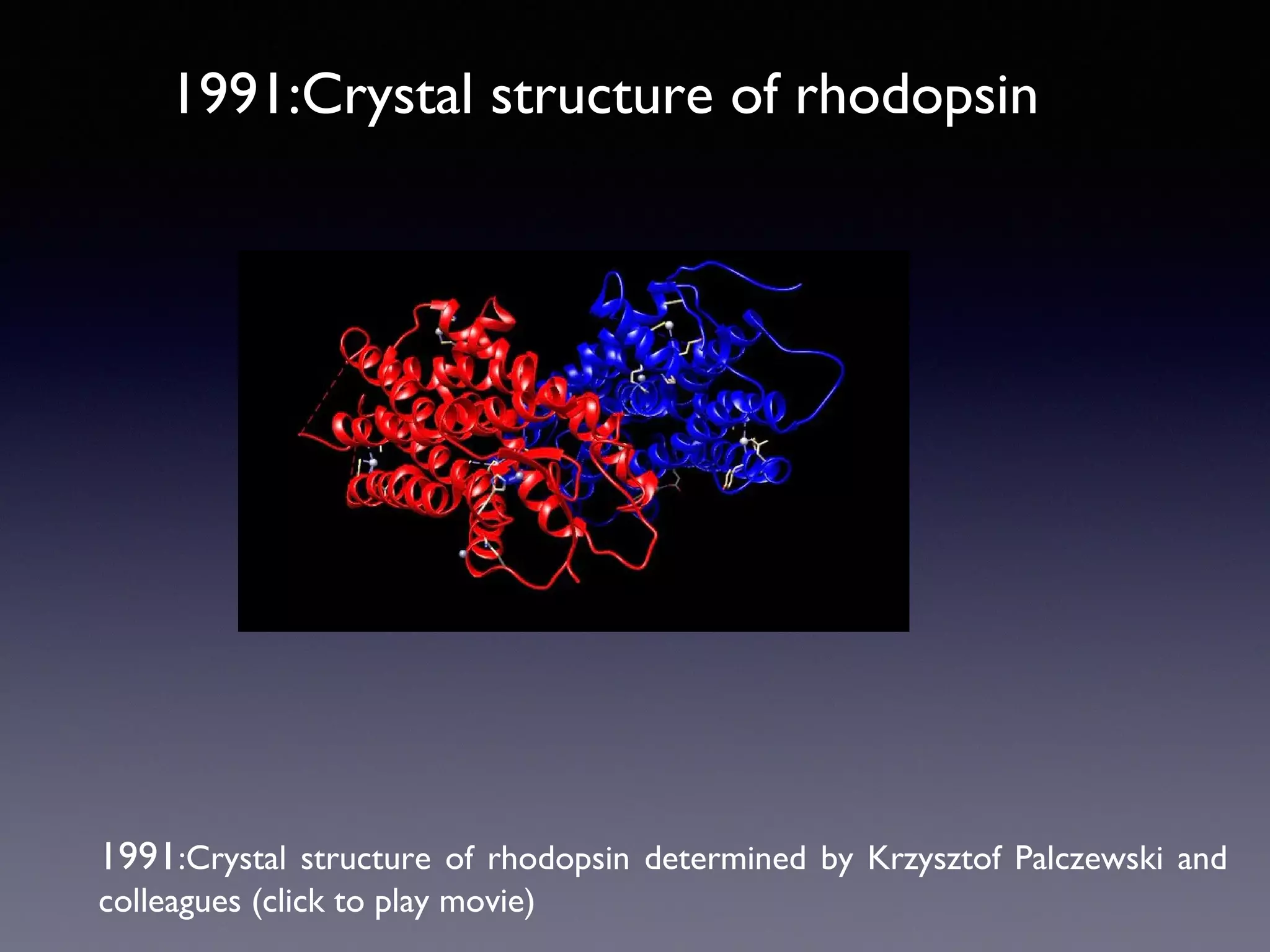
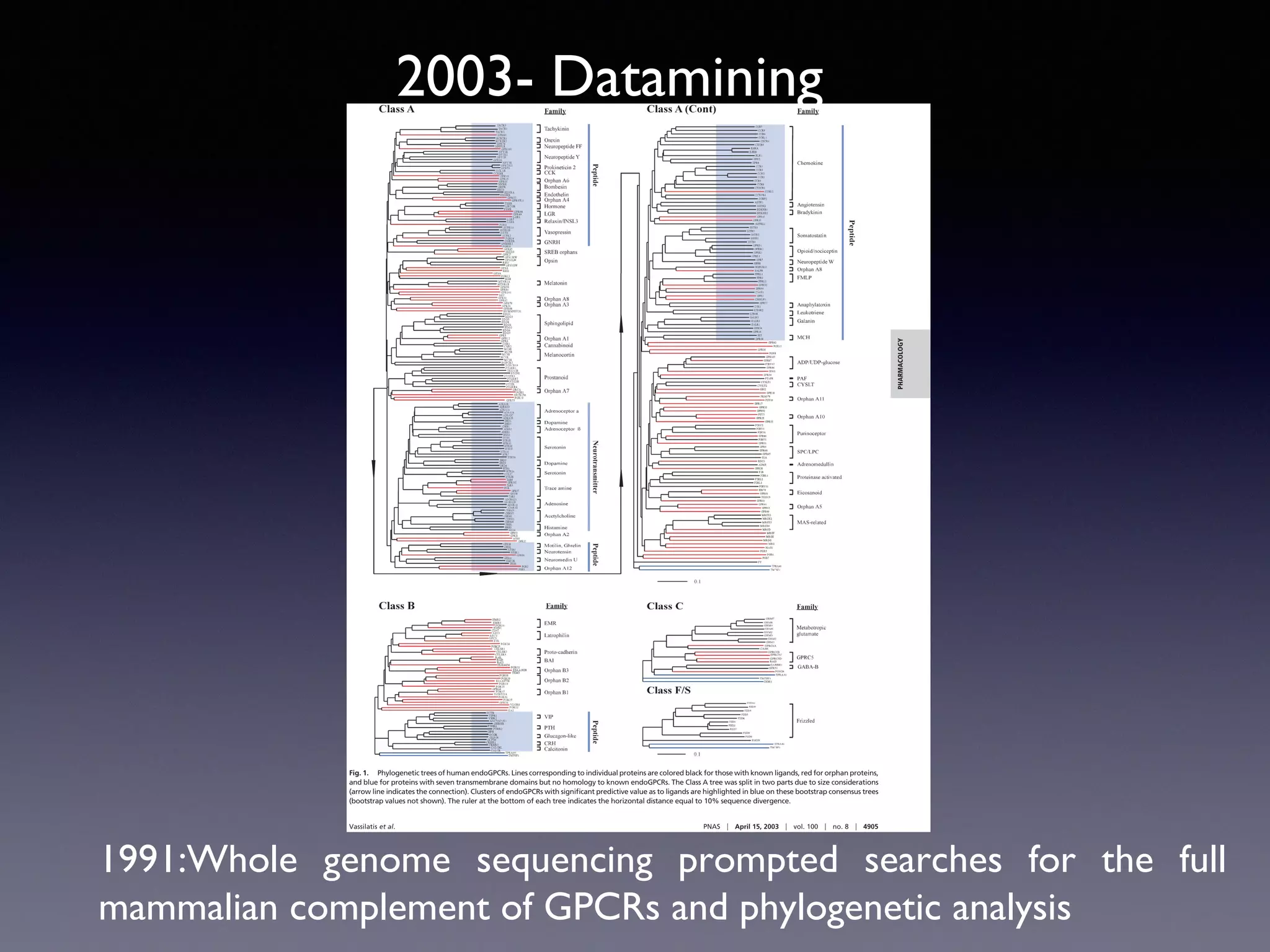
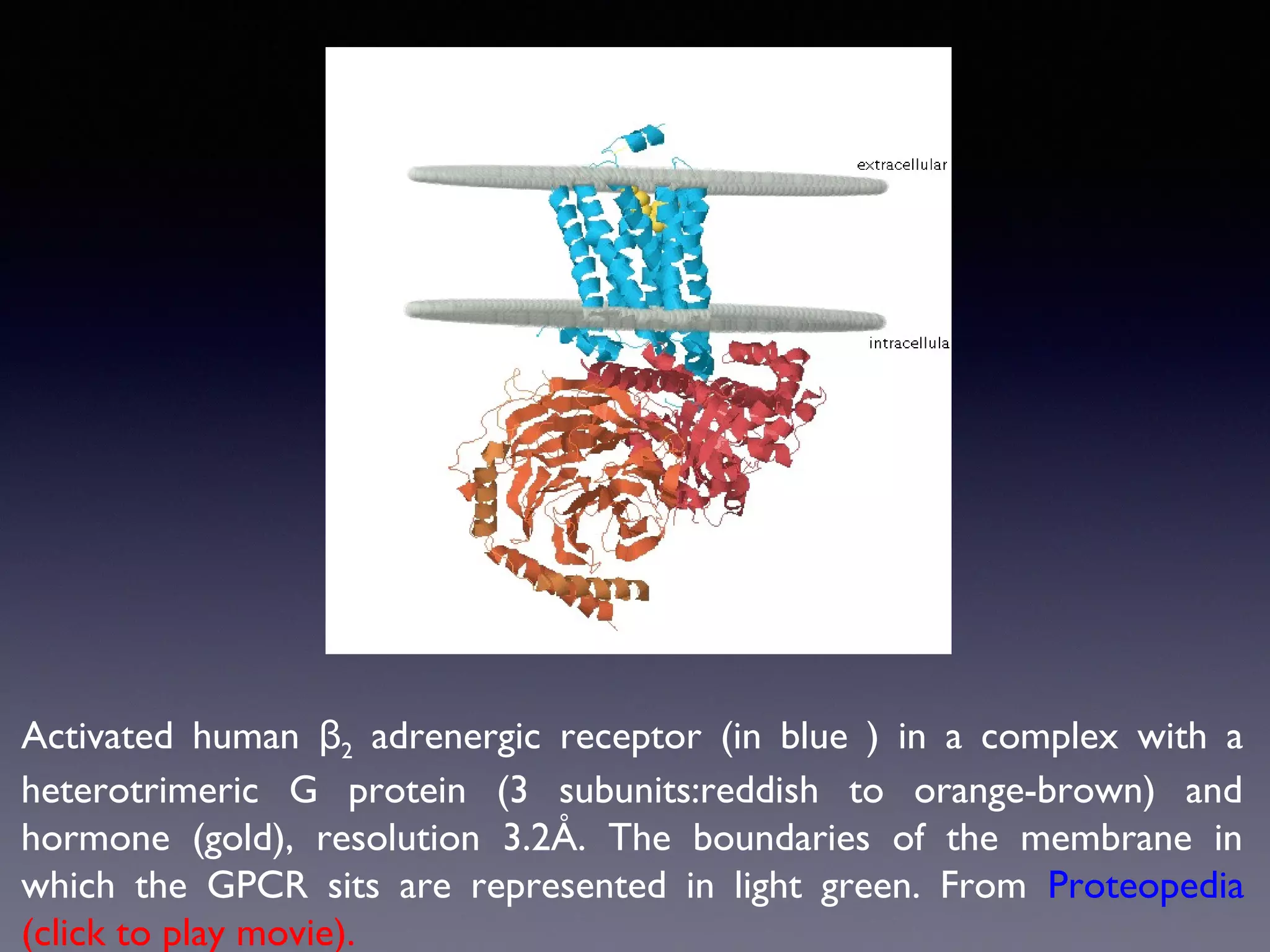
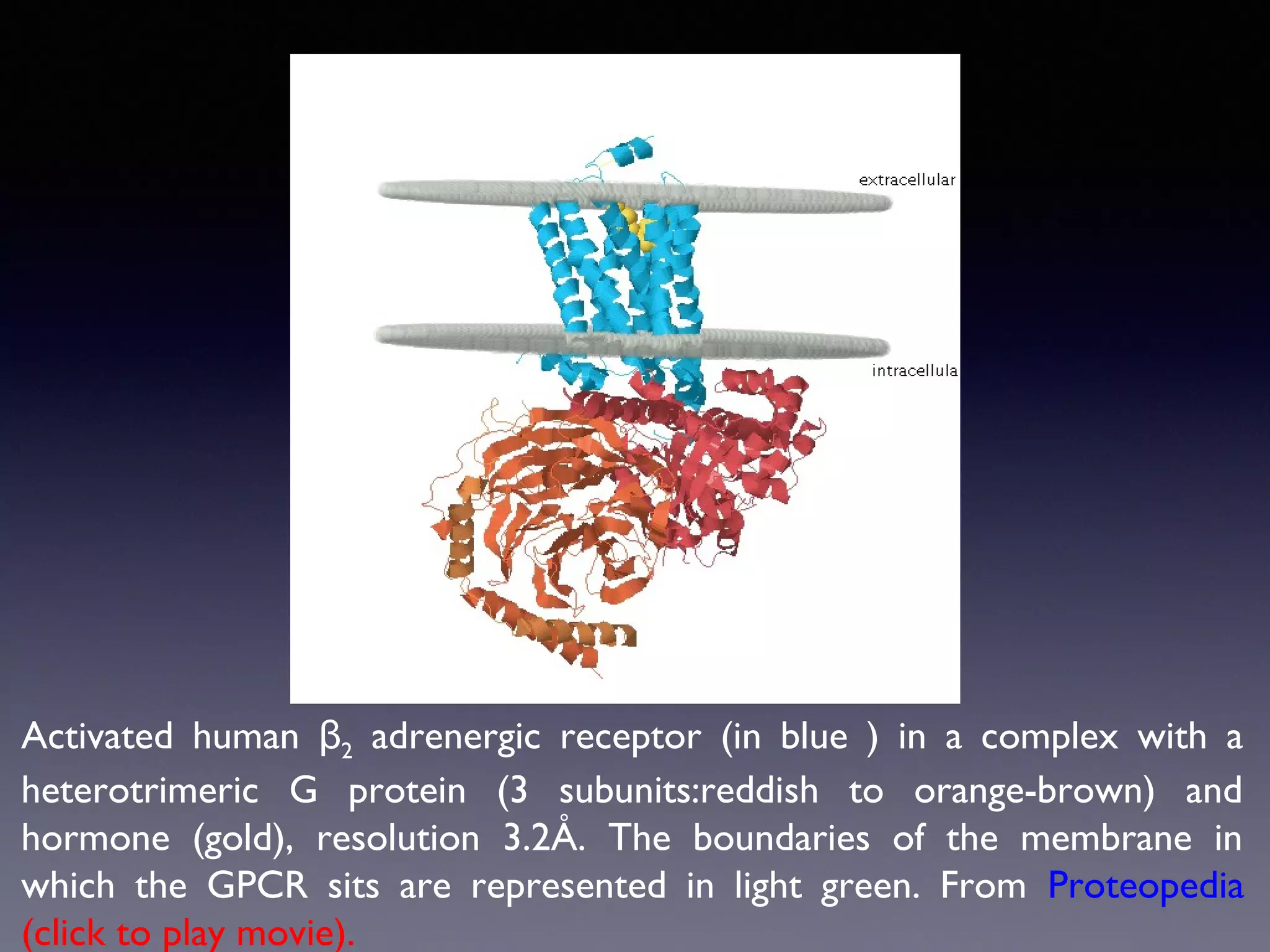
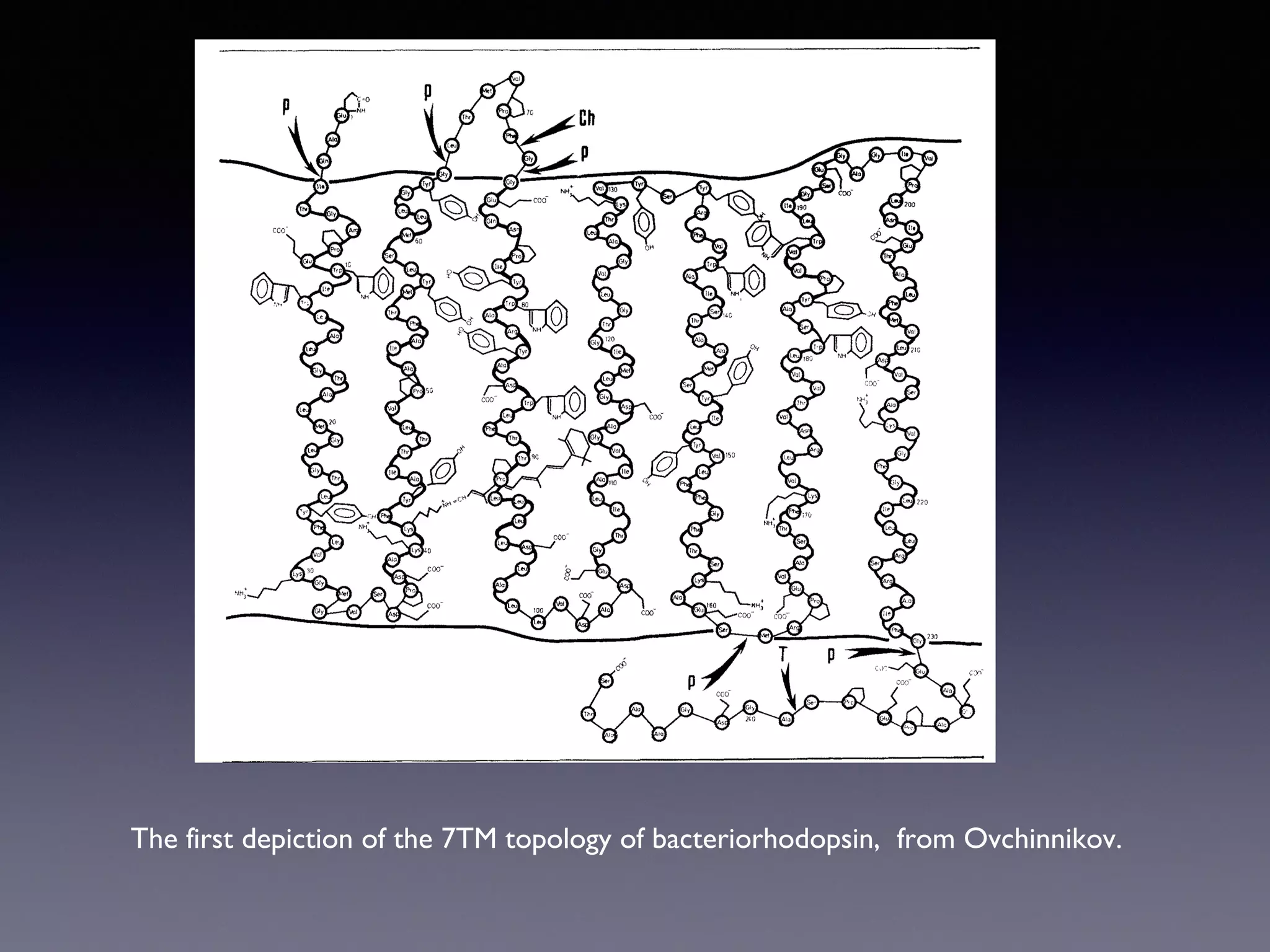
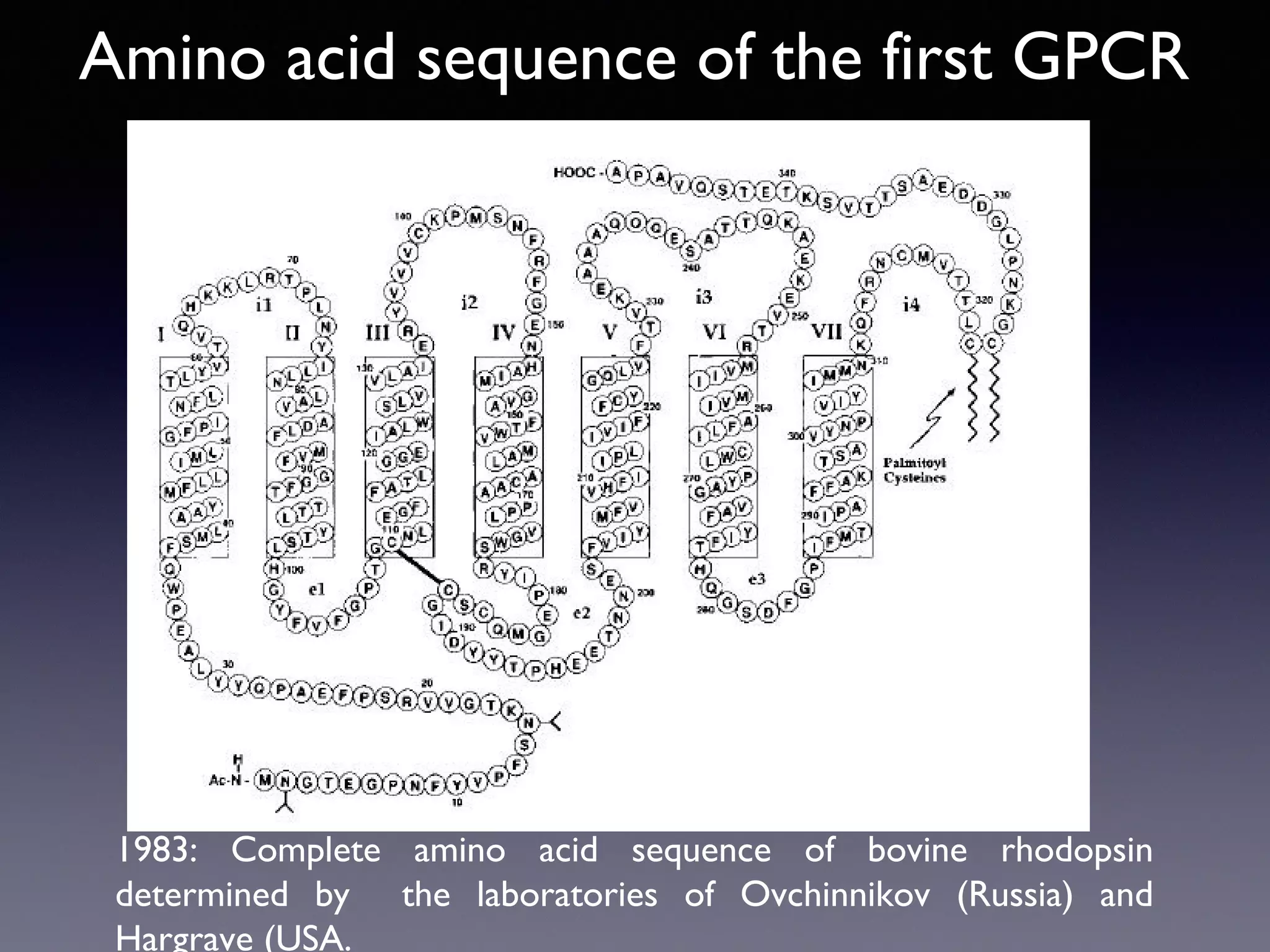
The document summarizes key developments in the structural characterization of G protein-coupled receptors (GPCRs), beginning with the 1975 structure of bacteriorhodopsin determined by Henderson and Unwin, showing its 7 transmembrane alpha helical structure. It then discusses important milestones such as the 1983 cloning of bovine rhodopsin, the first GPCR cDNA; the 1986 cloning of the beta-2 adrenergic receptor, the first non-sensory GPCR; the 1988 cloning of the 5-HT1A receptor, the first "orphan" GPCR to be deorphanized; and the 1991 crystal structure of rhodopsin, the first GPCR structure. The document concludes with the






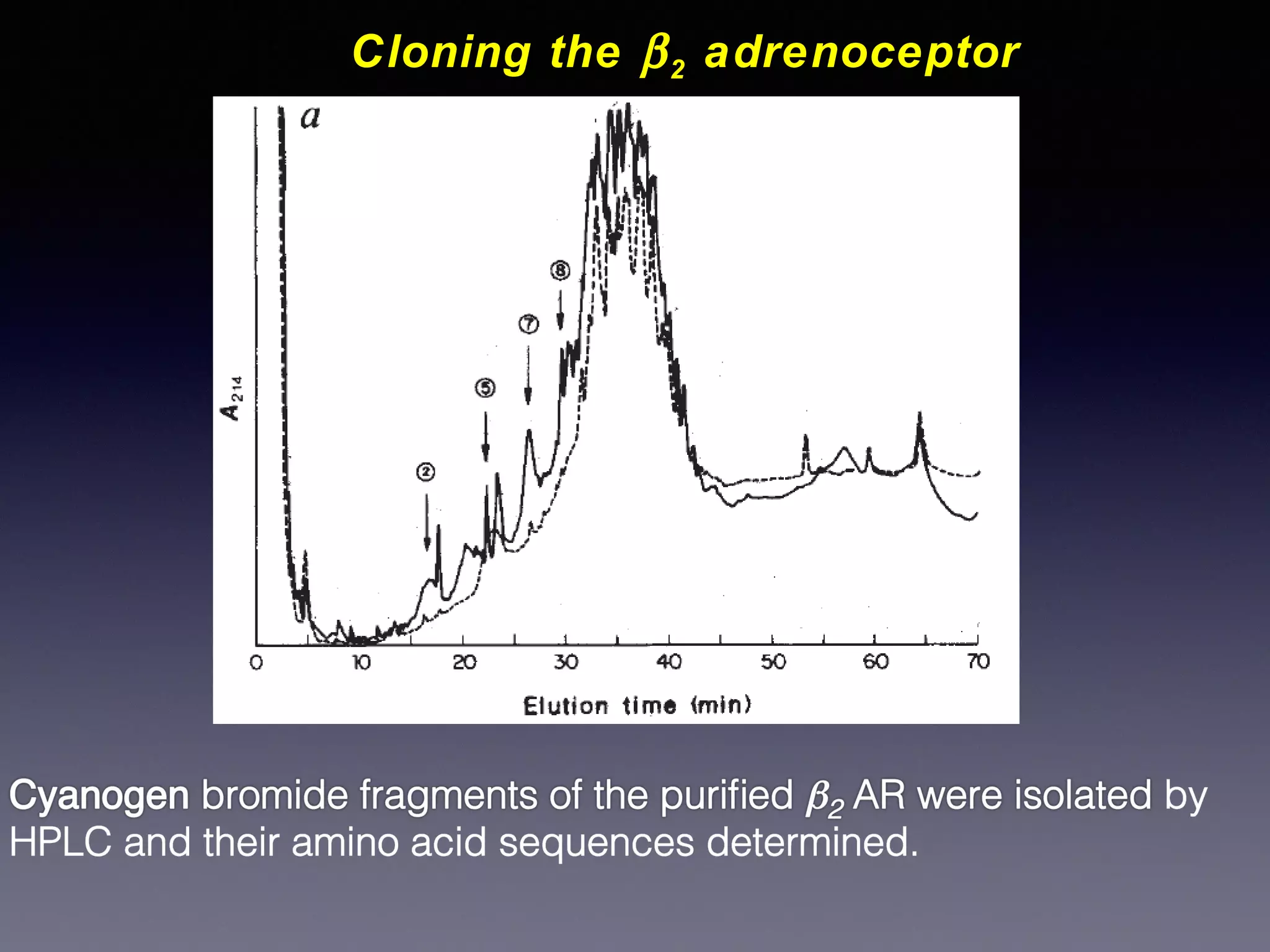
![Cloning the β 2 adrenoceptor
•
Receptor from hamster lung solubilised in detergent and purified by
affinity chromatography on alprenolol-sepharose
•
Progress of purification monitored by binding of [ 125 I]-cyanopindolol
•
Attempts to obtain amino acid sequence of the intact protein failed
•
Purified protein was subjected to chemical cleavage with cyanogen
bromide (CNBr), which cleaves proteins after every methionine
residue
•
Cyanogen bromide fragments were purified by HPLC and
sequenced](https://image.slidesharecdn.com/gpcr-20structures-20061213b-131210072834-phpapp01/75/Gpcr-structures-061213b-10-2048.jpg)